Utilizing Protecting Groups in Organic Synthesis
Synthesis of (R)-5-((31-methoxydotriacont-15-en-1-yl)sulfonyl)-1-phenyl-1H-tetrazole.
The 15-hydroxypentadecanoic acid methyl ester (2) was prepared from ω-pentadecalactone (1) by reaction with sodium methoxide in methanol at reflux temperature.
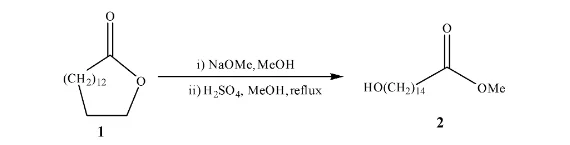
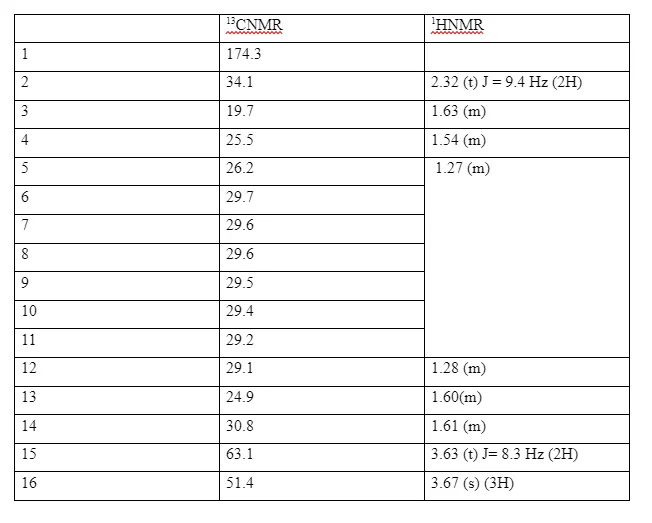
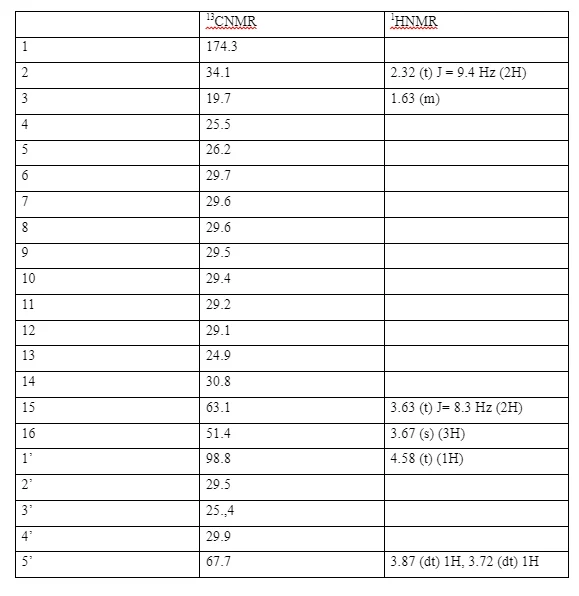
The 1H NMR spectrum of (2) showed a singlet at δ 3.66 OCH3 and a triplet at δ 3.63 (J 6.6 Hz) (CH2OH) together with a triplet at δ 2.3 with coupling constant 7.5 Hz for CH2 next to the carbonyl group. The 13C NMR spectrum showed a signal at δ 174.4 for the carbonyl carbon at δ 63.1 for the carbon next to the hydroxyl group (CH2OH) and a signal at δ 51.4 (OCH3) . In addition to these there were other carbon signals for the straight carbon chain assigned as follows δ The IR spectrum showed a broad band at 3298 cm-1 for the OH stretch and the broad peak at 1742 cm-1 for the C=O stretch.
The protection of the alcohol functional group
The use of protecting groups in organic chemistry reaction has been referred to as a a necessary evil. It’s use increases the number of steps in the synthesis by creating two additional steps which does not lead to the formation of the product. This also has an implication on the cost of synthesis in that it increases the cost, but its necessary because it masks reactive functional groups, through steps involving reagents which can react with the group, thus preventing the formation of side products which can complicate the synthesis. It also avails the protected functional group when it is needed to react. This is achieved through selective deprotonation reaction. Ethers are used as protecting groups for alcohols due their inert nature 1. The only potential reaction ethers undergo is hydrolysis under strongly acidic conditions to form alcohols. This makes them suitable protecting groups because a protecting group is supposed to be inert to the reaction conditions applied in the subsequent steps of the reaction before it is deprotected 2. The most commonly used ethers are tetrahydropyranyl and methoxymethyl ethers3.
Looking for further insights on Understanding Mycobacterial Structure? Click here.
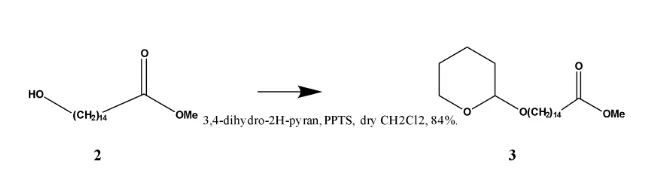
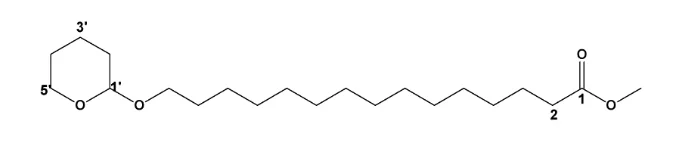
3, 4- dihydro- 2H-pyran undergoes an addition reaction across the double bond to form methyl 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecanoate. The reaction is catalysed with pyridinium_ p-toulenesulfonate (PPTS), a weakly acidic catalyst to prevent the possibility of hydrolysis of the ester functional group. The 13CNMR spectrum of (3) shows an oxymethine peak (O-CH) at δC 98.8 (C-1’) and an oxy-methylene peak (O-CH2) at δC 67.7 (C-5’), for the tetrahydro- 2H- pyran carbons and the corresponding proton chemical shifts, from 1HNMR spectrum were, δH 4.58 (t) ( J= 3.2 Hz, 1H) for the methine proton (H-1’) and δH 3.87 (dt) 1H, δH 3.72 (dt) IH for the two protons at (C-5’). The two protons are non-chemically equivalent, because the they are in a ring. This in addition with the proton and carbon-13 signals identical to those of compound (2) confirmed the attachment of the 3,4- dihyro-2H- pyran ring onto the alcohol end of the Methyl-15-hydroxypentadecanoate to form methyl 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecanoate. Unlike in the spectrum of (2) the two protons at H-15 are now not chemically equivalent due to the electronic environment created by the attachment of the tetrahydropyran ring. The rest of the carbon-13 and proton signals were assigned as shown in the table.
13CNMR and 1HNMR assignments of methyl 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecanoate.
Reduction of the ester functional group to a primary alcohol
Lithium aluminium hydride is used for the reduction in this case because it’s a strongest among the hydride reducing agents, since it is a strong reducing agent and it can reduce the less reactive ester functional group to a primary alcohol. The greater hydride character is because the Al-H bond is more polarised than other hydride reducing agents. Two equivalents of the reducing agent is used in this reaction. The aldehyde is first converted into an aldehyde which is then subsequently reduced. NMR spectroscopy was used to confirm the success of the reaction.
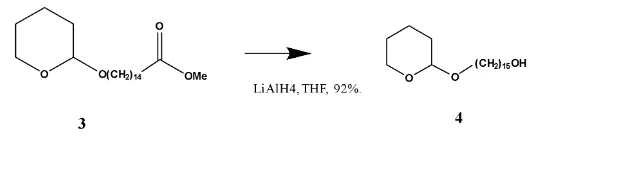
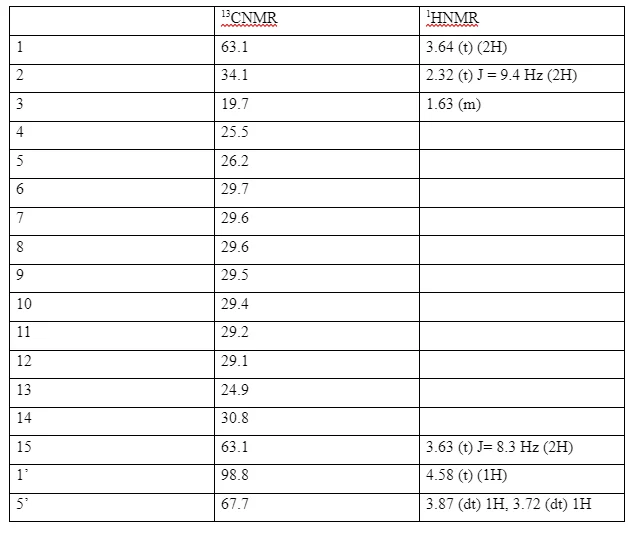
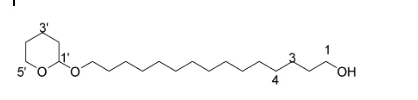
The 13CNMR spectrum of compound (4) showed no signal above 150 ppm indicating that the carbonyl carbon had been reduced. In addition to this there was no methoxy carbon and protons as was seen in the spectra of compound (3) above, and there was an additional oxymethylene carbon indicating the presence of a terminal hydroxyl group. This confirmed that the ester had been reduced to form a primary alcohol. The 13CNMR spectrum of compound (4) showed the presence of an oxymethine peak at δC 98.8 and an oxymethylene peak δC 67.7 assignhed to the carbons C-1’ and C-5’ of the tetrahydrofuran moiety, two other oxymethylene carbons δC 63.1 and δC 62.3 assigned to C-1 and C-15 respectively. This was further supported by the presence of a methine proton δH 4.50 (t) 1H, δH 3.87 (dt) 1H and δH 3.72(dt) 1H (H-5’) for the tetrahydro- pyran moiety. δH 3.53 (dt) 1H and δH 3.38 (dt) 1H (H-15) and δH 3.64 (t) 2H (H-1) for the methylene protons of the straight chain. This confirmed the structure of compound (4) to be 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecan-1-ol. The other assignments are shown in the table below.
13CNMR and 1HNMR assignments of 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecan-1-ol
Structure of compound (4)
Oxidation of the primary alcohol to an aldehyde
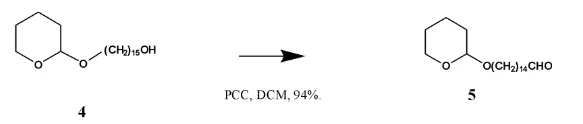
Pyridinium chloro-chromate (PCC) in the solvent dichloromethane (DCM) is used in order to selectively achieve the partial oxidation of the primary alcohol to aldehyde. The use of other chromium based oxidising agents in aqueous solutions leads to the formation of carboxylic acids instead. The reaction is under anhydrous condition to avoid the reaction of the aldehyde formed from reacting with water to form a dihydrate which can further be oxidised to give a carboxylic acid 4.
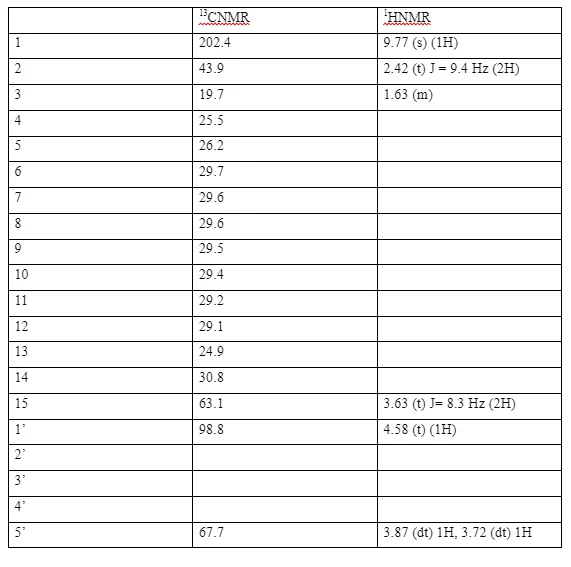
The 13CNM R spectrum of compound (5) showed a deshielded carbonyl peak at δC 202.4 typical of an aldehyde carbonyl carbon. This was further supported by the presence of a downfield shifted one-proton singlet at δH 9.77. In the 13CNMR spectrum of compound (5) there were only two oxymethylene carbon signals as opposed to three as in the spectra of compound (4), and in addition to the other signals seen in the spectra of compound (4), there was a methylene carbon at δC 43.9 (C-2) which is an sp3 carbon deshielded by the electronic environment of the aldehyde. 13CNMR and 1HNMR assignments of 15-((tetrahydro-2H-pyran-2-yl)oxy)pentadecanal
The formation of alkene through the Wittig reaction
The Wittig reaction is a powerful reaction for the formation of alkenes from carbonyl compounds (aldehydes and ketones) and phosphonium ylides5. The advantage of using the Wittig reaction is that streochemicall outcomes of the reaction can be determined through the choice of the ylide used. Unstabilised phosphorous ylides lead to the formation of Z-alkenes while stabilised phosphorous ylides form E- alkenes. The reaction forms only one double bond exactly where the carbonyl carbon was6. The Wittig reaction also employs mild reaction conditions5. In this reaction an ustabilised phosphorous ylide is used and a Z-alkene is formed. The reaction proceeds via a [2 +2] cycloaddition followed by a cycloreversion.

The 13CNMR spectrum of compound (6) showed the presence of an olefinic carbon signal at δC 129.9. This peak was for the two olefinic carbons because of the presence of symmetry in the molecule the two are chemically equivalent. The peak is also of high intensity indicating it is as a result of more than one carbon nuclei. In addition to these there were; a methoxy carbon δC 55.9 (C-33), two oxymethine peaks δC 98.8 (C-1’) and δC 76.9 (C-31), two oxymethylene peaks at δC 67.7 (C-5’) and δC 62.3 (C-1), a methyl group at δC 19.1 (C-32) and a set of methylene carbon signals. The 1HNMR spectrum of compound (5) showed the presence of signals at δH 5.36 (t) 2H for the chemically equivalent olefinic protons (H-15 and (H-16), δH 4.58 (t) 1H for the methine proton on the tetrahydropyran ring (H-1’), δH 3.88 1H and δH 3.76, δH 3.50 , δH 3.39 (H-), δH 3.28 1H for the methine proton (H-31), δH 3.32 (s) 3H for the methoxy protons (H-33). The presence of both proton and carbon signals for the olefin group indicated the success of the Wittig reaction. Additionally there was no carbonyl carbon signal indicating the aldehyde had reacted.
Deprotection of the alcohol functional group
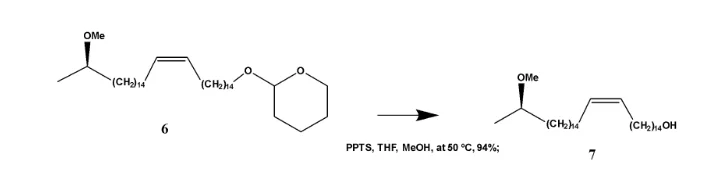
The tetrahydro-furan moiety which was used in step one to mask the primary alcohol is removed by acid hydrolysis. A weak PPTS is used in this reaction. In the 13CNMR spectrum compound (7) there is only one oxymethine carbon at δC 76.6 as compared to the spectrum of compound (6) where there were 2 oxymethine peaks on belonging to the tetra-hydropyranyl at δC 98.8. In addition to this only one oxymethylene carbon signal was present in the 13CNMR of compound (7) in comparison with compound (6), where they were two. This confirmed the cleavage of the tetrahydropyranyl which was used in step one as a protecting group for the primary alcohol.
The Mitsunobu reaction
This reaction involves the dehydrative coupling of an alcohol which could be a primary, secondary or tertiary alcohol to a pronucleophile (NuH)7. This dehydrative coupling is mediated by a reaction between a triaryl / trialkylphosponium and dialkylazodicarbonyl 8. As the reaction proceeds the azo species is reduced to a derivate of hydrazine and the phosphine is oxidised to phosphine oxide. The reaction generally proceeds with inversion of configuration in almost all cases when a secondary chiral alcohol is used7. Soeme suitable pronucleophiles for the Mitsunobu reaction include; (thio) carboxylc acids, amides, (thio) phenols and sulphonamides. These lead to the formation of C-S, C-N and C-O bonds. Typical Mitsunobu reagents include; diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD) and triphenyl phosphine (PPh3) 9. The DEAD and (PPh3) react to form a betaine with a pKa of 13. In order for this betain to deprotonate an acidic proton from the pro nucleophile, the pronucleophile must have a pKa of 11 or below as dictated by the pKa rule10. If the pKa is above 11, alkylation of the DEAD will occur instead. Solvents commonly used for the reaction include; THF, toluene, diethyl ether, dichloromethane and sometimes even polar such as DMF, ethyl acetate and acetonitrile are also used.
The synthetic scheme
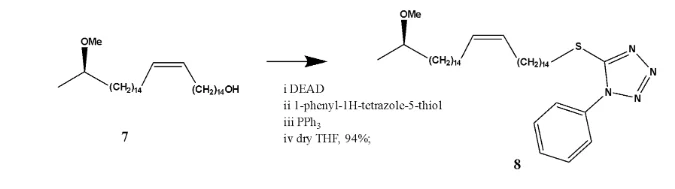
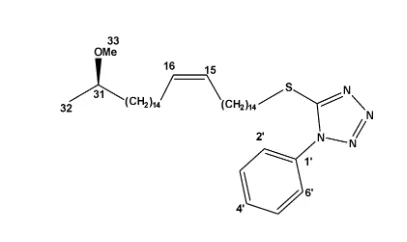
The 1HNMR spectrum of compound (8) showed the presence of five aromatic protons in an ABC spin system, at δH 7.61 (m) 2H assigned to (H-2’ and H-3’), δH 7.56 (m) 2H assigned to (H-4’ and H-6’) and δH 7.54 (m) 1H assigned to H-5’ confirming the presence of a phenyl substituent. This was further confirmed in the 13CNMR and DEPT NMR spectrum which showed 3 sp2 hybridised, methine carbon signals at δC 130.0 assigned to C-2’ and C-3’, δC 129.9 assigned to C-4’ and C-6’ and δC 129.8 assigned to C-5’. The peak at δC 123.9 in the 13CNMR together with the peak at δH 5.35 (dt) 2H confirms the presence of a C=C bond at C-15 and C-16. The 13CNMR spectrum of compound (8) also showed the presence of a methoxy carbon at δC 55.9 (C-33) with the corresponding protons resonating at δH 3.34 (s) 3H (H-33). In the 1HNMR spectrum of compound (8), methyl protons resonating at δH 1.13 (d) 3H (H-32) was evident with the corresponding carbon resonating at δC 19.2. The 13CNMR showed the presence of an oxymethine carbon δC methylene 76.7 assigned to C-31 with the proton attached to this carbon resonating at δH 4.33 (dd) 1H. peaks δC and δC , not seen in the DEPT spectrum indicating they are quartenary carbons assigned to phenyl carbon C-1’ and the tetrazoline carbon C-7’. The carbon chemical shift value of C-1 is substantially reduced since sulphur is less electronegative compared to oxygen. It’s chemical shift value is δC 36.3. Compound (8) is therefore (R)-5-((31-methoxydotriacont-15-en-1-yl)thio)-1-phenyl-1H-tetrazole , confirming the success of the Mitsunobu reaction.
Oxidation of (R,Z)-5-((31-methoxydotriacont-15-en-1-yl)thio)-1-phenyl-1H-tetrazole compound (7) to form (R,Z)-5-((31-methoxydotriacont-15-en-1-yl)sulfonyl)-1-phenyl-1H-tetrazole.
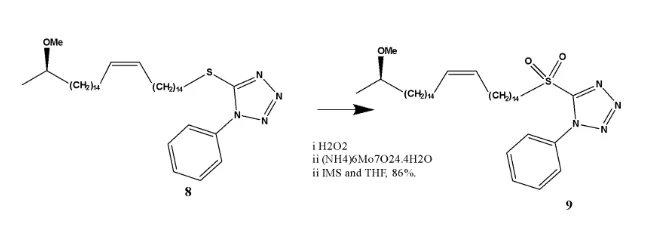

Hydrogen peroxide is the oxidising agent while the molybdenium complex is a catalyst. The 13CNMR spectrum of compound (9) is similar to that of compound (8) with the exception that the chemical shift of C-1, the carbon adjacent to the oxidised sulphur has become ore deshielded with a chemical shift of δC 56.0. Compound (9) is (R)-5-((31-methoxydotriacont-15-en-1-yl)sulfonyl)-1-phenyl-1H-tetrazole.
References
- Plante, O. J., Buchwald, S. L., & Seeberger, P. H. (2000). Halobenzyl ethers as protecting groups for organic synthesis. Journal of the American Chemical Society, 122(29), 7148-7149.
- Agoston, K., Streicher, H., & Fuegedi, P. (2016). Orthogonal protecting group strategies in carbohydrate chemistry. Tetrahedron: Asymmetry, 27(16), 707-728.
- Luo, X., Ma, X., Lebreux, F., Markó, I. E., & Lam, K. (2018). Electrochemical methoxymethylation of alcohols–a new, green and safe approach for the preparation of MOM ethers and other acetals. Chemical Communications, 54(71), 9969-9972.
- Reiter, D., Frisch, P., Szilvási, T., & Inoue, S. (2019). Heavier Carbonyl Olefination: The Sila-Wittig Reaction. Journal of the American Chemical Society, 141(42), 16991-16996.
- Liu, M. G., Liu, N., Xu, W. H., & Wang, L. (2019). Tandem reaction strategy of the Passerini/Wittig reaction based on the in situ capture of isocyanides: One-pot synthesis of heterocycles. Tetrahedron, 75(18), 2748-2754.
- März, M., Chudoba, J., Kohout, M., & Cibulka, R. (2017). Photocatalytic esterification under Mitsunobu reaction conditions mediated by flavin and visible light. Organic & Biomolecular Chemistry, 15(9), 1970-1975.
- Dobbs, A. P., & McGregor-Johnson, C. (2002). Synthesis of fluorous azodicarboxylates: towards cleaner Mitsunobu reactions. Tetrahedron letters, 43(15), 2807-2810.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



