ANA Testing: Diagnostic Advances
Report 1
Introduction
The ANA (antinuclear antibodies) testing is a very important step to diagnose many SARD (systemic autoimmune rheumatic diseases). In the recent times, the ANA’s early detection and its relevance as the SARD’s predictors has been demonstrated. The diagnostic and the predictive ANA value show the precise detection’s importance pertaining to the autoantibodies. The IIF (indirect immunofluorescence) on HEp-2 (human epithelial type 2) cells still has the regards of being the ANA screening’s gold standard method as it has very high degree of sensitivity (Yoo et al., 2017). However, the lacking of specificity limits the IIF assay’s diagnostic accuracy. In overcoming certain drawbacks of the traditional techniques of IIF, several efforts are made in developing an automated IIF assay (Satoh et al., 2007). Additionally, SPAs (solid phase assays)have been making advances that provides the possibility of alternatives to ANA and IIF testing. Despite the continuation of the employment of the traditional IIF method, the new techniques have been incorporated in certain laboratories as ANA detection’s routine assays.
Antinuclear antibodies (ANA) patterns
The pattern of staining may have the relation with a number of auto antibodies (e.g. association of speckled pattern with Mi-2, SSB/La, SSA/Ro, and U1-RNP) technical skills required for their recognition, and albeit high titres of a certain pattern’s presence has sufficiency with respect to the diagnosis that does not have the need for a confirmatory test, such as centromere pattern. Specific to kinetochore and centromere functions are the anti-centromere antibodies. Their occurrence has been in certain autoimmune diseases, often in limited systemic scleroderma and in scleroderma’s diffuse form. In many cases, there is requirement of the second test, which is more specific in identifying the antibody’s target antigen.


The IIF-ANA test’s utility and sensitivity have variation in accordance to the SARD type. The variations of some antigens’ cellular concentration can be producing a disparity in the performance of assay (Smith et al., 2017). For instance, the underrepresentation of the SSA/Ro antigen in the HEp-2 cells, limits the sensitivity in the antibodies’ detection against this antigen (Kidd et al., 2005). In a cell line that is modified and known as HEp-2000 contains the SSA/Ro expression has been increased genetically with the addition of complementary DNA of transfected human 60 kDa Ro(Hoffman et al., 2002). As IIF substrate, the usage of HEp-2000 cells enhances the ANA assay’s sensitivity by almost 10 percent (Cozzani et al., 2008).
Dig deeper into Overview of Baby Clothing with our selection of articles.

Another key consideration is IIF test’s false positive results. The apparently healthy individuals up to 30 percentages at a titer of 1:40 can be ANA “positive” (Hernandez Ramirez and Cabiedes, 2010). The DFS (dense fine speckled) represents another issue in relation to the anti-DFS70 antibodies. Considering that these antibodies have higher prevalence in healthy subjects compared to the patients of SARD, the anti-DFS70 antibodies’ clinical significance does not have clarity. It has been suggestive that they could be natural or protective autoantibodies.
Association with diseases
The autoantibodies detection against ANAs has been very important in the SARD diagnosis such as SjS (Sjögren’s syndrome) and SLE (systemic lupus erythematosus), MCTD (mixed connective tissue disease), SSc (systemic sclerosis), and IIMs (inflammatory myopathies). Thus, ANAs testing in the patients’ differential evaluation is a logical first step when there is suspect of systemic autoimmune etiology (Cabiedes and Nunez-Alvarez, 2010). SARDs’ timely diagnosis is exigent because of the overlapping symptoms’ wide spectrum. Moreover, while the ANAs’ frequency is the highest with SARD patients, the organ-specific autoimmune disease patients also have these antibodies. The example of autoimmune disease include Hashimoto’s thyroiditis, autoimmune liver diseases, and also other diseases such as cancer, some infections, and advanced age. Therefore, the testing of the ANA with individuals having low probability of pretest for a SARD can be the causation of the undue concern (Akmatov et al., 2017).
Tests
The testing of ANA is used commonly in assessing the chance of the diagnosis of the SARD, with the coming of the relevant information from the antibodies’ identification to specific intracellular targets. While the underlying immunological mechanisms with these interactions are understood rather poorly, the ANAs’ presence is usual representative of self-antigens’ abnormal responses, an important autoimmunity feature (Liang et al, 2009). In the routinely done evaluations of clinical laboratory, the general categorizations are on the basis of the homogeneous, nucleolar patterns, centromere, and speckled recognition. While the use of the ANAs have been the part of certain SARDs’ diagnosis (e.g. SjS, MCTD, and SLE); their presence might be serving as a key diagnostic support for others (juvenile arthritis, secondary SjS, IIMs, and SSc ()( Cho and Gregersen, 2011) .
The ANA’s recognition in the staining pattern with the use of HEp-2 cell substrate can be a helping hand to determine the presence of the most likely autoantibodies along with the suggestion of the possible clinical association for the specificities known. In this respect, a positive screening pattern of ANA can be a guiding hand to confirm the testing and can have usefulness to elucidate a specific clinical prognosis or diagnosis. ANA tests have been positive for virtually all SSc cases, with some associated patterns having multiple antigenic targets that have been critical for clinical management and stratification (Cavazzana et al., 2008). For instance, the SSc (anti-U3-RNP, anti-Ku, scleroderma (Scl)/ antipolymyositis (PM), anti-Th/To, and anti-RNAP III) and ANAs target proteins found in the nucleolus and nucleus. The association of these antibodies with some SSc manifestations includes survival prognosis and organ involvement (Fujimoto et al., 2016) . Of the several SSc antibodies, three major specificities (anti-RNAP III antibodies, anti-topo I, and ACA) have been recommended in the criteria of classification. Antibodies to U3-RNP and Th/To also have had the consideration of specific for SSc, while Ku, U1-RNP, and PM/Scl are generally detectable in the overlap syndromes patients, such as SSc and SLE or SSc and PM. In the recent time, specific ANAs have more usefulness in the IIMs’ evaluation (Fujimoto et al., 2016). While the specificities and patterns of IIMs and ANAs are diverse to a large extent, certain autoantibodies’ identification has usefulness to both distinguishing and diagnosing between myositis’ subtypes and to monitor or predict the developing of the additional clinical manifestations, which includes cancer risks and organ involvement. The development of the further suggested tests have been in detecting ANAs in the serum of the patients. This includes Western blot (immunoblotting), immunodiffusion, RIA (radioimmunoassay), MIA (microsphere multiplex immunoassays), ELISA (enzyme-linked immunosorbent assay), immunoperoxidase staining, microarray, immunoelectrophoresis, dot blot, and IIF.
References
Akmatov, M. K., et al. (2017) ‘Anti-nuclear autoantibodies in the general German population: prevalence and lack of association with selected cardiovascular and metabolic disorders: findings of a multicenter population-based study’, Arthritis Res Ther, 19:127.
Cozzani, E., Drosera, M., Siccardi, M., Babbini, G. and Parodi, A.(2008) ‘Indirect immunofluorescence on HEp-2000 substrate is a sensitive method for detecting anti-Ro/SSA antibodies in patients with lupus erythematosus and/or photosensitivity’, J Rheumatol, 35(7):1320-2.
Cho, J.H. and Gregersen, P.K. (2011) ‘Genomics and the multifactorial nature of human autoimmune Disease’, N Engl J Med, 365: 1612–23.
Cavazzana, I., Ceribelli, A., Quinzanini, M., Scarsi, M., Airo, P., Cattaneo, R., et al. (2008) ‘Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases’, Lupus, 17:727–32.
Hernandez Ramirez, D.F. and Cabiedes, J. (2010) ‘Immunological techniques that support the diagnosis of the autoimmune diseases’, Reumatol. Clin, 6(3):173–177.
Hoffman, I. E., Peene, I., Veys, E. M. and De Keyser, F. (2002) ‘Detection of specific antinuclear reactivities in patients with negative anti-nuclear antibody immunofluorescence screening tests’, ClinChem, 48(12):2171–6.
Satoh, M., Chan, E. K., Sobel, E. S., Kimpel, D. L., Yamasaki, Y., Narain, S., et al. (2007) ‘Clinical implication of autoantibodies in patients with systemic rheumatic diseases’, Expert Rev Clin Immunol, 3(5):721–38.
Report 2
Introduction
The positive serum EmA having almost 100 percent association with coeliac disease makes around 10-20 percent of the patients being untreated with coeliac disease remaining negative for serum EmA (Hill, 2005). The patients having negative serum EmA and histological lesions that are on the borderline, on the other hand, are treatable with gluten-free diet (GFD). Here, the possibility always exists for the coeliac disease’s false diagnosis. Data is suggestive of the possibility of whether EmA negativity has been in relation to the histological or specific clinical course of coeliac disease that is conflicting. The studies mostly are suggestive of the EmA negativity having common association with the mild histological lesions that is contradictory of the notion that for early‐stage coeliac diseaseEmA is a marker without villous atrophy (Walker‐Smith et al., 1990).
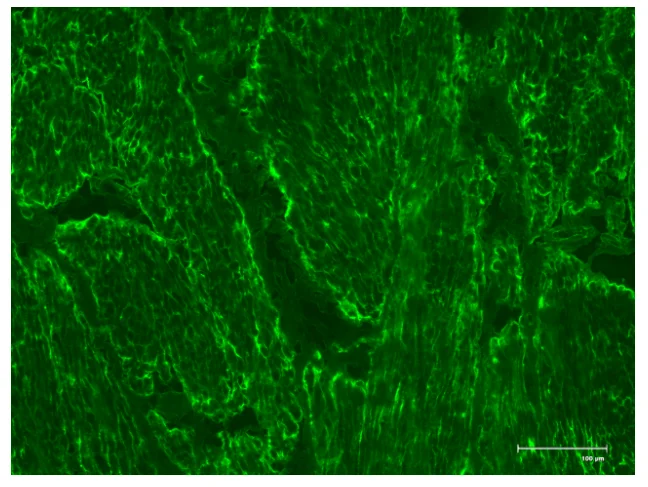
In the samples of coeliac disease with EmA‐binding patterns have been proven to be Type 2 transglutaminase (TG2) ‐targeted exclusively and the TG2 and EmA antibodies having a good correlation.TG2 is cancer cell survival protein which survives in closed and open conformations. It is evident that the produced coeliac autoantibodies are in small‐bowel mucosa (Marzari et al., 2001).
Findings
Among the patients having coeliac disease, the patients to the extent of 15 percent had negative serum EmA, and a small percentage of them having IgA‐deficient. Therefore, the patients with negative EmA and with coeliac disease comprised the study group. DQ8 or HLA DQ2 has been detected in less than half of the patients with sample available (DQ8 in 1 and HLA DQ2) (Salmi et al., 2006). Amongst the patients with negative EmA and with coeliac disease, men to the tune of 59 percent and the median age have been higher than patients with positive EmA (Salmi et al., 2006). Abdominal symptoms have been more common significantly compared to the EmA‐negative group. Three patients with negative EmA and coeliac disease have been found to have EATL (enteropathy‐associated T cell lymphoma) that is detectable as the coeliac disease diagnosis at the same time (Salmi et al., 2006). With regards to HLA DQ2, there have been two patients having it. All these patients had crypt hyperplasia and proximal small‐bowel villous atrophy having compatibility with coeliac disease, while on diet contains gluten. Moreover, two patients out of them had small‐bowel biopsy taken before, and two to six years before EATL and coeliac disease diagnosis. During that time, both showedcrypt hyperplasia and partial villous atrophy of althoughcoeliac disease diagnosis has been overlooked. Overall, 27 percent of the patients with negative EmA and 4 percent of the patients with positive EmA with coeliac disease have had death after coeliac disease diagnosis (Salmi et al., 2006). No observed differences have been found between EmA‐positive and EmA‐negative patients with coeliac disease. The mucosal IgA deposits of small bowel in colocalisation with extracellular TG2 have been detectable in all EmA‐positive (n = 17) and EmA‐negative (n = 18) (Salmi et al., 2006) patients examined with coeliac disease. The intestinal IgA deposits’ intensity had no correlation with the mucosal lesion’s severity, which is villous height: crypt depth ratios. For instance, three patients with negative EmA having coeliac disease and with 1.5 height:crypt depth ratios had 2.5+ to 3+ intensity IgA deposits (Korponay‐Szabo et al., 2004). In obtaining the IgA deposits’ TG2 specificity’s direct evidence, more experiments had been conducted. The pericryptal IgA and small‐bowel mucosal subepithelial deposits with TG2 in both EmA‐positive and EmA‐negative patients having coeliac disease had been unchanged in the post treatment with 0.5–1 M KSCN and citrate buffer. Contrastingly, amongst the deposits with IgA, the significant decrease has taken place in eight samples and virtual disappearance in five samples when treatment of these sections and been with additional chloroacetic acid, with which the TG2 is removed from the fibronectin binding sites. The detectable TG2 amount also decreases in parallel, where IgA in epithelial cells’ brush border remained unchanged essentially. There had been similar effect with chloroacetic acid in EmA‐positive and EmA‐negative samples (Salmi et al., 2006).
Tests
Serology
EmA samples of the serum IgA‐class are measurable in identical settings. An immunofluorescence method which is an indirect one is usable as substrate with human umbilical cord; a 1:⩾5 dilution has been subject to consideration to be positive. The negative and positive controls had been inclusive in every test batch (McMillan et al., 1991). The serum IgA‐class TG2 antibodies’ assessment was carried out with the use of ELISA.
The serologic tests that can be conducted in screening coeliac disease are the followings
Antigliadin antibody
This test was the first among the serologic tests having relatively low specificity and sensitivity making it unsuitable in screening coeliac disease. AGA are antibodies of anti-food protein and not does not indicate of any autoimmune reactions (Lindenmann, 1984).

Antireticulin antibody
The next serologic test developed was this assay. This also is unsuitable for screening coeliac disease as there is availability of more sensitive tests. ARA is usually used in coeliac disease evaluation, although they lack optimal specifications and sensitivities for routinely done diagnostic use (Lindenmann, 1984).
Antiendomysial antibody
The antiendomysial antibody is a test having high sensitivity and specificity for coeliac disease. This assay has the requirement of laboratory technicians in assessing immunofluorescence, which leads to potential variability in interobserver to interpret the results of the tests. In this test, there is generation of antigens by the tissue transglutaminase effect modifying glutamine residues in glutamic acid’s proteins (Lindenmann, 1984).
Anti-tTG antibody
The antigens were discovered against which there have been the formation of endomysial antibodies, such as enzyme tTG. The human tTG, with recombinant technology, has the availability for commercial use with the testing cost subsiding considerably. The measurement of tTG antibody is done in most hospitals now rather than the antiendomysial antibody (Lindenmann, 1984).
Small‐bowel mucosal inflammation and morphology
The seven specimens of biopsy on endoscopy have been taken from duodenum’s distal part. The processing was done with five, along with staining of the eosin and haematoxylin and has been under study under light microscopy. The interpretation of the specimen has been in accordance with the Marsh’s criteria(Rostami et al., 1999). The further classification of the Marsh III lesion have been into three subgroups: (a) Marsh IIIa, whose indication has been partially severe; (b) subtotal of Marsh IIIb; and (c) totally villous atrophy of the Marsh IIIc. Additionally, mucosal histology’s study have been more objective with the depth ratio of height:crypt and had been under the determination from the biopsy samples that are well oriented from multiple sites. The consideration of a ratio that is less than 2 will have compatibility with coeliac disease.
Small‐bowel mucosal IgA deposits targeted by TG2
In the studies conducted previously, the patients with positive EmA and having coeliac disease possess situ IgA deposits in vivo on TG2 in the mucosa of small bowel. When there is elution of IgA from the tissues, TG2 of purified nature is targeted by it both in western blot and ELISA (Korponay‐Szabo et al., 2004). The used methods in these tests have been on the basis of experiments conducted previously in detecting antibodies that are TG2‐specific in tissue section in situ by their TG2 colocalisation when immune fluorescence double labeled it (Telci and Griffin, 2006).
References
Hill, I.D. (2005) ‘What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations?’, Gastroenterology, 128: S25-S32.
Marzari, R., Sblattero, D., Florian, F. et al (2001) ‘Molecular dissection of tissue transglutaminase autoantibody response in celiac disease’, J Immunol, 66, 4170–4176.
McMillan, S. A., Haughton, D. J., Biggart, J. D. et al (1991) ‘Predictive value for coeliac disease of antibodies to gliadin, endomysium, and jejunum in patients attending for jejunal biopsy’, BMJ, 303, 1163–1165.
Rostami, K., Kerckhaert, J., Tiemessen, R. et al (1999) ‘Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice’, Am J Gastroenterol, 4, 888–894.
Walker‐Smith, J. A., Guandalini, S. and Schmitz, J. et al (1990) ‘Revised criteria for diagnosis of coeliac disease’, Arch Dis Child, 65, 909–911.
Salmi, T. T., Collin, P., Korponay‐Szabó, I. R. et al., (2006) ‘Endomysial antibody‐negative coeliac disease: clinical characteristics and intestinal autoantibody deposits’, Gut,. 55(12): 1746–1753. .
Telci. D. and Griffin, M. (2006) ‘Tissue transglutaminase (TG2)–a wound response enzyme’, Front Biosci, 11: 867–882.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



