Comparative Analysis of Developmental Therapeutics Programs
DISCUSSION
Developmental Therapeutics Programs (DTPanti-cancer )'s screening program generates a large amount of data stored in a computerized database. Compare analysis is a method of comparing items and determining their similarities and differences (1). The value of the living beings tumor cell line assay as a discovery tool has significantly increased thanks to the COMPARE algorithm. The most recent in vitro-screen, which is used to evaluate the efficacy of synthetic and organic ingredients, took several years to develop and was released in April 1990. A total of 59 tumor tissue cells from humans have been divided into disease subpanels. Each month, the screen evaluates about 1,000 molecules and natural substance extracts at maximum capacity (3).The decision to not change the assay technique or cell lines used in the screen for an extended time allowed vast numbers of molecules to be evaluated under similar circumstances. COMPARE analyzes the resulting data. For those engaged in related research or seeking healthcare dissertation help, understanding these methodologies can provide valuable insights. The NCI accession number of a probe or "seed" compound is used to identify the compound a so-called (NSC number).
COMPARE ranks the entire archive of tested components in terms of how similar their cytotoxicity is to that of other compounds in the database (1). The Pearson correlation coefficient indicates how closely the pattern resembles that of the seed. According to the COMPARE algorithm, compounds near the top of the list may have a mechanism of action similar to the seed compound. It was proposed in 1985 that a screen could find cell-type-specific agents with clinical activity against solid tumors by looking for them (6). The realization that in vitro histology has a poor correlation with patient care is beginning to emerge, but the sequence of cell membrane responsiveness and drug reaction in cell lines correlates with molecular goal affirmation.
Compare analysis of NCS compounds
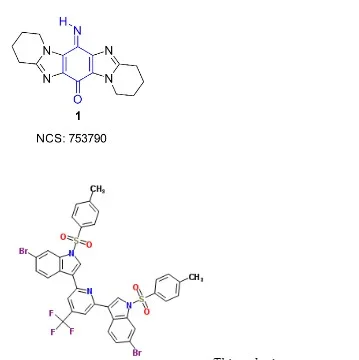
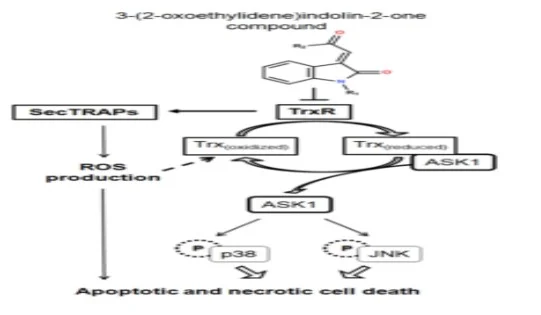
Thioredoxin is a family of small redox proteins found in all living things. It's involved in a number of crucial biological processes, including redox signaling. TXN and TXN2 genes encode thioredoxins in humans. The thioredoxin (Trx)-thioredoxin reductase (TrxR) system is important for maintaining cellular redox balance, and Trx is overexpressed in a number of cancers. TrxR inhibition is a key strategy in the development of anti-cancer drugs. Pleurotin is a well-known irreversible TrxR inhibitor that is a natural product (3).
Benzo[1,2,4]triazin-7
![Benzo[1,2,4]triazin-7](https://www.dissertationhomework.com/img/nk193.webp)
The cytotoxicity data for benzo[1,2,4]triazin-7-ones showed a very strong correlation (Pearson correlation coefficients 0.8) to pleurotin using the National Cancer Institute COMPARE analysis (3). Although the parent compound 1,3-diphenylbenzo[1,2,4]triazin-7-one was more cytotoxic against cancer cell lines, a new 3-CF3 substituted benzo[1,2,4]triazin-7-one showed submicromolar inhibition of TrxR (Fagan et al., 2016). Different types of reversible inhibition of TrxR were observed with benzo[1,2,4]triazin-7-ones, and cyclic voltammetry revealed characteristic quasi-reversible redox processes. Cytotoxicity was found to be strongly dependent on substitution at the 6-position of the 1,3-diphenylbenzo[1,2,4]triazin-7-one ring in cell viability studies.
Bassett et al., 2018 discovered benzotriazinone 6 as a minor byproduct during production 1,4-dihydro-7-methoxy-1,3-diphenyl-1,2,4-benzotriazin-4-yl from diphenyl-nitrilimine and 4-methoxyphenyliminotriphenylphosphorane in 1969. Later, in 1980, attempted to make benzotriazinone 6 directly by reacting 1,4-dihydro-1,3-diphenyl-1,2,4-benzotriazinyl (Blatter's radical) with oxygen in the presence of active charcoal (3). However, both routes produced very low yields, preventing further research into this quinonimine. Investigation of the chemistry of this potentially useful quinonimine was done after discovering that it was possible to obtain benzotriazinone 6 in multigram quantities (10). The dark purple color of triazin-7(1H)-one (6) is due to a strong input of a zwitterionic impedance form.
The formulation of ylidenemalononitrile 11 was regarded in an effort to create a "chromophore" with a higher wavelength absorption, possibly extending into the near-infrared.
Ylidenemalononitriles can also be used as scaffolds for the synthesis of a variety of other heteroarenes (5). All efforts to consolidate malononitrile with benzotriazinone 6 resulted in complicated, highly colored reaction solution from which no steady product could be secluded. As a result, alternative methods such as having to treat the benzotriazinone 6 with other dicyanomethylene sources were used.
Recrystallization of ylidenemalononitrile 11 produced dark green plates with a melting point of 306–311 C (CHCl 3) and a deep blue-green solution with a lmax (DCM) of 702 nm (log e 3.08), indicating a highly conjugated and possibly charge-separated system (2). Attempts to increase the yield of this reaction by using more TCNE equivalents or extending the reaction time resulted in a decrease in yield. When TCNE was replaced with tetracyanoethylene oxide (TCNEO), similar yields were obtained (5).

Further chemistry on the ylidenemalononitrile 11 was not considered worthwhile due to the low yields. Nonetheless, the failure of malononitrile to condense onto the carbonyl at C-7 and the absence of any identifiable n(C O) stretch in the IR spectrum of quinonimine 6 supported the charge-separated resonance form's strong contribution (10). As a result, it's unlikely that TCNE reacts with benzotriazinone via a concerted 2 + 2 cycloaddition, but rather that the benzotriazinone 6 reacts with the highly electrophilic TCNE in a stepwise manner to give the zwitterion 12, which then cyclizes to give the spirocyclic oxetane 13, which then eliminates the unstable carbonyl cyanide to give the ylidene 11.
Additionally, at around 20°C, benzotriazinone 6 was reacted with PCl3(1 equiv.) in POCl 3 to produce the 7-chloro-1,3- diphenylbenzo[e][1,2,4]triazin-1-ium (5). The cation was then treated with aniline (3 equiv.) to produce a deep blue compound with a mass spectrum molecular ion peak of m/z 374 Da, which could be N-(1,3-diphenylbenzo[e][1,2,4]triazin- 7(1H)-ylidene)benzenamine. The 1 H NMR was complex after chromatographic purification, indicating mixtures that could not be separated. This project is still ongoing.
The survival of benzo[1,2,4]triazin-7-ones and 1,2,4-benzotriazinyl (Blatter-type) radical precursors in cells is characterized, with correlations to 2,2,6,6-tetramethyl-1-piperidinyloxy radical precursors (TEMPO) (2). The benzo[1,2,4]triazin-7-ones were several orders of magnitude less cytotoxic than the steady free - radical. The formulation and examination of two new pyrid-2-yl benzo[1,2,4]triazin-7-ones are defined, with the 1,3-substitution from phenyl to pyrid-2-yl increasing cytotoxic effects against most cancer cell lines, as evidenced by one-dose testing at the National Cancer Institute. The NCI's three-dose testing data showed very strong correlations to the naturally occurring anti-cancer compound pleurotin, according to a COMPARE analysis (9). COMPARE is a program that compares cytotoxicity data from different compounds and allows for quantitative expression as Pearson correlation coefficients. Compounds were also assessed using an independent MTT assay, which was compared to data generated by the SRB assay data.
Ca2+ signaling in cancer cells has traditionally been studied in terms of cellular membranes Ca2+ penetrable cell signaling and their regulatory agencies. In comparison, very few studies looked at how proteins that regulate endoplasmic reticulum (ER) Ca2+ homeostasis might be remodeled in cancer (7). In responding to activating stimuli, the ER is the primary intracellular store for releasable Ca2+. The sarco/ER Ca2+ ATPase (SERCA) pumps, which vigorously trap cytosolic Ca2+ into the ER lumen, and inositol triphosphate synapses (IP3R), which discharge ER Ca2+ in reaction to IP3mobilizing agonists such as ATP, are the two major proteins that regulate ER Ca2+ homeostasis in epithelial cells.
A variety of cancers have abnormal calcium (Ca2+) signaling and associated regulatory proteins like Ca2+ channels (8). Cancer cells may alter their intracellular Ca2+ signaling machinery to favor tumorigenic processes, allowing them to survive and proliferate indefinitely (5). When compared to normal prostate tissues, prostate cancers have higher expression of Ca2+ channels such as transient receptor potential vanilloid 6 (TRPV6) and ORAI3 channels. Colorectal and blood cancers have been linked to abnormal SERCA expression and/or activity (9). These changes could be used to track treatment for cancer. Indeed, mipsagargin, a prostate-specific epithelium antigen-based prodrug that targets the SERCA pumps, is currently being tested in solid tumors. Protooncogenes like the Bcl2 family of antiapoptotic proteins, which lower ER Ca2+ levels by increasing ER Ca2+ 'leak,' can also affect ER Ca2+ signaling (2). Aside from these more well defined mechanisms of ER Ca2+ store regulation, proteins like the neuronal calcium sensor indirectly modulate ER Ca2+ signals.
Neuronal calcium sensor 1 is a 22kDa Ca2+ sensor protein with EFhands that is structurally similar to calmodulin. NCS1 has a myristoyl group at the N terminus that enables Ca2+ to attach to protein molecules and cellular membranes. NCS1 interacts with IP3R, increasing the likelihood of it opening (4) NCS1 silencing reduces IP3mediated Ca2+ signals mediated by ATP in neuroblastoma cells and endothelin in cardiomyocytes (9). NCS1 regulates neurotransmission activity, which is important for learning, memory, and synaptic plasticity, and is widely expressed in adult neuronal cells (2). Despite NCS1's role in Ca2+ homeostasis regulation and its link to breast cancer, no studies have looked into its role in intracellular Ca2+ signaling in breast cancer cells (4). The relationship between NCS1 expression and breast cancer molecular subtypes is also unknown. For the first time, it show that NCS1 expression is elevated in the molecular subtype of basal breast cancer. It was established that silencing NCS1 with siRNA reduced unstimulated basal Ca2+ influx in basal breast cancer cells (2). NCS1 silencing increased doxorubicin-induced breast cancer cell death, a phenomenon mirrored by the Ca2+ store refilling channel ORAI1 silencing.
Continue your exploration of Life with Hemi Spatial Neglect with our related content.
Despite assurances that NCS1 is linked to paclitaxel therapeutic response (7,8), it's unclear whether this is linked to changes in Ca2+ signaling in breast cancer cells. In pancreatic and liver cancer models, chemosensitization was achieved by inhibiting SOCE and suppressing Ca2+ influx (2). As a result, we tested whether silencing ORAI1 and NCS1 enhances the effects of doxorubicin, a commonly used chemotherapy in the treatment of basal or TNBCs. Using EdU staining, we first determined whether silencing of NCS1 and ORAI1 promoted the antiproliferative effects of doxorubicin.
The DU-145 cell line was significantly more cytotoxic to all three stable free radicals tested than the MCF-7 cell line. To the cancer cell lines tested, benzotriazin-4-yl radicals were significantly less cytotoxic than their oxidation products, benzo[1,2,4]triazin-7-ones. Using the MTT assay, pyridyl-substituted benzotriazin-7-ones had submicromolar cytotoxicity comparable to 1,3-bisphenylbenzo[1,2,4]triazin-7-one 1 (4). Because of the variable DTP-NCI one-dose testing cytotoxicity profiles, they were chosen for five-dose testing. Despite the overall greater cytotoxicity of the pyrid-2-yl-substituted compounds compared to 1after one-dose testing, COMPARE analysis revealed very strong correlations to pleurotin (2). Complete one- and five-dose data for can be found in the Supplementary Information accompanying this research. The NCI sampling for subsequent three-dose screening formed significant components used in the NCI COMPARE algorithm to determine closely matching cytotoxicity profiles. The COMPARE analysis made cytotoxicity comparisons with the NCI's vast database of over 250,000 synthetic compounds much easier. The Pearson product-moment correlation coefficient (0 to 1) describes the degree of similarity between two cytotoxicity profiles, with values above 0.5 considered strong (12). The Pearson correlation coefficient of 1 is comparable.
With cytotoxicity profiles similar to the irreversible TrxR inhibitor pleurotin, the 1,3-substitution in the benzo[1,2,4]triazin-7-one scaffold from Ph to pyrid-2-yl did not appear to change the compound's mechanism of action. The cytotoxicity of the 3-substituted 1-phenylbenzo[1,2,4]triazin-7-ones had a very strong correlation (Pearson correlation coefficients of 0.8) to the naturally occurring antibiotic and anti-cancer agent pleurotin, according to the National Cancer Institute (NCI) COMPARE analysis (12). The anti-cancer activity is thought to be due to thioredoxin reductase (TrxR) inhibition, with the 3-Ph substituted benzotriazinones showing strong reversible mixed and uncompetitive inhibition. Evidence that scaffold analogues were multi-target inhibitors of Alzheimer's disease prompted our anti-cancer research (2). At room temperature, Blatter-type (benzotriazin-4-yl) radicals are treated with manganese dioxide in dichloromethane to produce benzotriazinones.
Continue your exploration of Community mental health interventions with our related content.
Reference
Apasu JE, Schuette D, LaRanger R, Steinle JA, Nguyen LD, Grosshans HK, Zhang M, Cai WL, Yan Q, Robert ME et al (2019) Neuronal calcium sensor 1 (NCS1) promotes motility and metastatic spread of breast cancer cells in vitro and in vivo . FASEB J 33, 4802–4813. [PMC free article] [PubMed] [Google Scholar]
Azimi I, Milevskiy M, Chalmers S, Yapa K, Robitaille M, Henry C, Baillie G, Thompson E, Roberts‐Thomson S and Monteith G (2019). ORAI1 and ORAI3 in breast cancer molecular subtypes and the identification of ORAI3 as a hypoxia sensitive gene and a regulator of hypoxia responses. Cancers 11, 208. [PMC free article] [PubMed] [Google Scholar]
Bassett JJ, Bong AHL, Janke EK, Robitaille M, Roberts‐Thomson SJ, Peters AA and Monteith GR (2018) Assessment of cytosolic free calcium changes during ceramide‐induced cell death in MDA‐MB‐231 breast cancer cells expressing the calcium sensor GCaMP6m. Cell Calcium 72, 39–50. [PubMed] [Google Scholar]
Berridge MJ (2016) The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96, 1261–1296. [PubMed] [Google Scholar]
Bittremieux M, Parys JB, Pinton P and Bultynck G (2016) ER functions of oncogenes and tumor suppressors: modulators of intracellular Ca2+ signaling. Biochim Biophys Acta 1863, 1364–1378. [PubMed] [Google Scholar]
Boeckel GR and Ehrlich BE (2018) NCS‐1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta 1865, 1660–1667. [PMC free article] [PubMed] [Google Scholar]
tp.cancer.gov 2021, Developmental Therapeutics Program, Available at: https://dtp.cancer.gov/ [Accessed on: 27 November 2021]
dtp.cancer.gov 2021a, Screening Procedures, Available at https://dtp.cancer.gov/databases_tools/docs/compare/compare_methodology.htm [Accessed on: 27 November 2021]
dtp.cancer.gov 2021b, COMPARE Analysis, Available at: https://dtp.cancer.gov/databases_tools/compare.htm [Accessed on: 27 November 2021]
Fagan, V., Bonham, S., Carty, M.P., Saenz-Méndez, P., Eriksson, L.A. and Aldabbagh, F., 2016. COMPARE analysis of the toxicity of an iminoquinone derivative of the imidazo [5, 4-f] benzimidazoles with NAD (P) H: quinone oxidoreductase 1 (NQO1) activity and computational docking of quinones as NQO1 substrates. Bioorganic & medicinal chemistry, 20(10), pp.3223-3232.
Humphreys, R.K., Puth, M.T., Neuhäuser, M. and Ruxton, G.D., 2019. Underestimation of Pearson's product-moment correlation statistic. Oecologia, 189(1), pp.1-7.
Obilor, E.I. and Amadi, E.C., 2018. Test for significance of Pearson’s correlation coefficient. International Journal of Innovative Mathematics, Statistics & Energy Policies, 6(1), pp.11-23.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



