Understanding Cytokines and Interleukins
Introduction
Background:
Cytokines are important messengers of the immune system that play major roles in generating the appropriate immune response against any pathogen that enters in the body (Namai et al. 2020). Interleukins (ILs) are groups of cytokines that modulate and control the immunological behavior of cells. ILs are involved in stimulating the appropriate immunological response to any antigen inside the body thereby causing inflammation and cell mediated charges (hormonal changes and changes in biological parameters). Cytokines also control the immune response to autoantigens. Autoantigens are the complex of protein, that are recognized and targeted by the cell mediated immune response, in which cytokines play crucial roles in detecting the autoantigens and destroy them to maintain proper immune response against autoantigens (Al-Eitan et al. 2020). Some members of the Interleukin 1 family acts as important promoters of inflammation by increasing its secretion into the circulation while the body is infected by pathogens. IL-1 superfamily consists of 11 different members (Grauers Wiktorin et al. 2021). IL-1 alpha and IL-1 Beta are the two most active immunological members of the IL-1 family (Boldrup et al. 2021). These molecules play crucial roles in mediating and regulating many inflammatory processes in the human body. Interleukin 1 receptor antagonist (IL-1RA) is encoded by the IL-1RN gene (Dandona et al. 2018). IL-1Ra is also termed as IL-1 inhibitor, as it binds to the interleukin 1 receptor (IL-1R) surface thereby preventing the IL-1 to send any kind of intracellular signal. IL-1RA plays a crucial role in preventing the proinflammatory effect of IL-1 alpha and IL-1 beta molecules (Namai et al. 2020). Additionally, Interleukin 1 receptor antagonist (IL-1RA) also modulates and control various IL1 associated inflammatory and immunes response (Boldrup et al 2021). For those seeking to delve deeper into these mechanisms, healthcare dissertation help can provide valuable insights. IL-1 alpha is an important cytokine that is activated and produced mainly by endothelial cells, macrophages, epithelial cells and neutrophils.

After activation of the IL-1 alpha it binds to the Interleukin 1 receptor (IL-1R) thereby generating the proper immunological signal (Yadav et al. 2017). On the other hand, IL- 1 beta is the major pro-inflammatory cytokine that plays a crucial role in managing many inflammatory responses inside the body (Al-Eitan et al. 2020). IL-1 beta is strongly associated with monocyte activation while receiving the signal from the inflammatory agents such as bacteria, virus and other pathogens (Porrit et al. 2020). Monocytes are the types of white blood cells or leucocytes which undergo differentiation into dendritic cells and macrophages (Robuffo et al. 2017). Dendritic cells and macrophages are associated with three important immunological functions such as production of cytokines, phagocytosis and antigen presentation. Therefore, after activation of monocytes these three procedures are initiated which develop potential immune response inside the body against the pathogens (Smith et al. 2018).
During an inflammation, an external, endogenous or microbial stimulus is received by the body which then causes the activation of the proinflammatory cytosolic signaling molecule, which is termed as inflammasome (Dandona et al. 2018). inflammasome can be defined as the cytosolic multiprotein oligomer which is responsible for activating and initiating an inflammatory response. The activation of inflammasome leads to maturation, cleavage and secretion of pro cytokines molecule or protein which is termed as caspase 1 (Farooq et al. 2021).
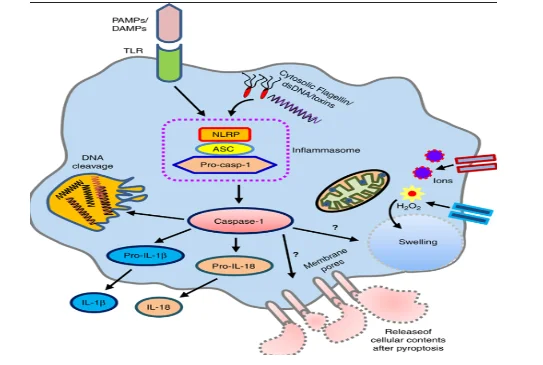
Caspase-1 protein is the major cytokine activator in the immune system (Parker et al. 2020). On activation caspase 1 causes activation of proinflammatory cytokines such as pro-IL1B and pro-IL18. During this activation are differentiated into IL1B and IL18 that play important roles in maintaining proper immune response inside cell (Mortensen et al. 2021). The, matured IL-1B and IL-18 cause the activation of other immunological factors such as PRRs (pattern recognition receptors), the pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) (Dandona et al. 2018). Additionally, through the production of B cells and immunoglobulin, IL-1B generates a proper inflammatory response against the inflammation thereby mediating different inflammatory syndrome.
Research aim:
This research project aims to develop a Luminex assay to detect anti- IL-1RA and anti-IL-1 beta antibodies
Overview of materials and techniques:
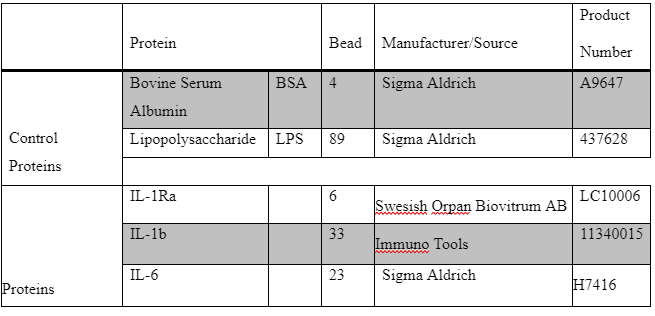
During this study the coupling of Luminex based anti-IL-1RAand anti IL-1B antibodies serology was conducted. in this stage a covalent chemical coupling of the recombinant IL-1B and IL-1Ra Luminex beads was performed. Then a verification and evaluation of the successful coupling procedure was done using the appropriate monoclonal antibodies. During preparing the colored beads for this assay, the mixture of the colored beads is pre-coated with the analyte-specific antibody. Analyst specific antibody enables colored beads to be easily detected on the spectrum as apparent colored band ().
The Luminex assay was performed to detect anti- IL-1RA and anti-IL-1B antibodies using a Luminex assay. A Luminex assay is widely used and highly effective immunological assay that assists the researchers to detect and measure different analytes that are present in the mixture of antibodies. (Farooq et al. 2021). This is a bead-based immunoassay that enables researchers to detect up to or more than 100 immunological analytes in the sample.
A cohort of patients which suffer from severe inflammation was selected for this research project. serum samples from the healthy control donors were used. In this process a sample containing the covalent chemical coupling of the recombinant IL-1B and IL-1Ra antibodies was used. The coupling procedure had been used to make a multiplex mixture of IL-1B and IL-1Ra antibodies for this assay (Porritt et al. 2020). The spike control sera were used to check the successful setting up as well as validation of the IL-1B and IL-1Ra in the monoplex and multiplex mixture (Dosh et al. 2019). A multiplex assay is the immune assay in which the magnetic beads are used in detecting different analytes in a single experiment (Mortensen et al. 2021). The spike control sera were proved to be highly useful in this study that enabled the research study to detect different charterers of the multiplex of IL-1B and IL-1Ra (Smith et al. 2018). These characteristics are linearity of mixture, the colour and texture of the beads, the dynamic range of this assay, interference, sensitivity, and the intra assay precision.
Methods:
Activation of carboxylate microsphere:
Each microsphere sets which is 1ml (1.25 X 10 7 beads) was transferred into a correctly labelled non-stick RNAse free 2.0ml microfuge tubes. Then, all tubes was centrifuge for 4 min at 13000g. Extended fine tip Pastette was used carefully to remove the supernatant from the opposite side from the bead pellet.
Each microsphere was washed twice with 500ul of 100mM MES buffer (PH6.0) followed by 30 second vortex and bath sonicate for the 60 seconds and centrifuge for 4 min at 14000g.
At this stage beads was activated by adding 100ul of S-NHS (50mg/ml in 100mM MES PH6.0) and 100ul EDC (50mg/ml in 100mM MES PH6.0) into each beads. Then all tubes was vortexed for 30 second and rotated for 30 min at room temperature in the dark.
After 30 min all activated beads was washed twice with 750ml of PBS PH 7.4 followed by vortex 30 second and centrifuge at 14000g for 4 min. Then activated beads was re-suspended with 100ul of PBS PH7.4 and vortex and bath sonicated for 30 seconds.
Coupling of covalent of IL-1B and IL-1Ra:
After activation, 400μl containing 50μg of protein, was added to the activated beads and the volume was adjusted to 500μl with PBS (pH 7.4). All tubes were then placed on a rotator at room temperature for 2 hours in the dark. The protein-coupled beads were then washed twice in 1ml of PBS (pH 7.4). Each bead set's concentration adjusted to 3x106 beads/ml using storage buffer.
Quality control of coupled beads
A 96-well filter plate (1.2 μm pore size; Millipore) was used to test quality control of beads. Each beads were tested separately with previously coupled BSA (LC10004-01; region 4). 50 μL mixture of IL-1Ra and BSA beads, IL-6 and BSA beads and [IL-1b and BSA] beads were separately added to pre-wetted wells at a final concentration of 4.8∙104 beads/mL. BSA-coupled beads were included as a negative control. Liquid was removed by aspiration using a vacuum manifold (15.6 kD; FB70155; Fisher Brand).
A 1:3 serial dilution of 2 μg/mL α-IL-1Ra-biotin antibody (brand and cat#) 2 μg/mL α-IL-1b-biotin antibody (brand and cat#) )and 2 μg/mL α-IL-6-biotin antibody (brand and cat#) ) were added in duplicate to the relevant beads as a detection antibody. The plates were incubated for 1h at RT on an orbital shaker (500-600 rpm; Eppendorf Thermomixer comfort) in the dark.
The liquid was removed by vacuum aspiration, wells were washed three times with 120 μL wash buffer and liquid was removed by vacuum aspiration. 50 μL Streptavidin-phycoerythrin (Strep-PE; final concentration 1 μg/mL; 554061; BD Pharmingen) was added. A blank with only Strep-PE was included. The plate was incubated for 30 min at RT on the shaker (500-600 rpm) in the dark and washing was repeated as described above. Beads were re-suspended in 120 μL wash buffer per well. Fluorescence was measured with a LUMINEX 200 System (gate setting: 5000-25,000; high target).
Luminex immune assay:
The analyser calibration was carried out regularly according to manufacturer’s instructions. The quality control material sera had been diluted at 1:100 blocking buffer (0.8% polyvinylpyrrolidone (PVP), 10mm PBS. 1% BSA, 0.5% polyvinyl alcohol (PVA) and 0.05% sodium azide). The sera mixture was incubated for the next 60 minutes in dark at room temperature. Gentle shaking was done continuously during the incubation (Boldrup et al. 2021). Then 40μl of each microsphere is added to the 5 ml blocking buffer.
Then the mixture is vortexed for the 30 second, 50μl of the mixed beads were added to each well. Then the removal of the buffer from the beads was done carefully using the vacuum manifold before the adding of the control samples into the well. After 60 minutes at 370C on shaker an automated plate washer is used to wash the plate for 3 times.
Use of biotinylated antibodies
Here the biotinylated antibodies that are highly specific to the targeted antibodies (IL-1B and IL-1Ra) are added to the beads thereby forming an antibody-antigen bindings. Phycoerythrin (PE)-conjugated streptavidin is also added in this mixture which then binds to the biotinylated antibodies.
The plate was incubated for 30 min at 370C on a shaker after adding 50 μL Streptavidin-phycoerythrin. After washing the plate, the beads were then suspended on the buffer and then kept on the orbital shaking agent for 5 minutes to keep the beads on suspension. Fluorescence intensity was measured with a LUMINEX 200 System.
Statistical analysis
Statistical analyses were performed by comparing (group) means with the Mann-Whitney U test. After measuring the fluorescence with the LUMINEX 200 System, standard curves were created with Bioplex Manager 6.1 plotting concentration vs fluorescence intensity (Fig X)
Result:
IL-1Ra and IL-1B proteins successfully coupled to beads
A binding assay was performed to validate the coupling of IL-1Ra and IL-1B to beads. A 1:3 serial dilution of 2 μg/mL α-IL-1Ra Abs was added to the beads and the fluorescence was measured using the LUMINEX 200 System. A titration curve was created as displayed in figure 1 and 2.

Titration curve with mean fluorescence intensity (MFI) against log α-IL-1b Abs concentration in μg/mL. Error bars represent standard deviations.
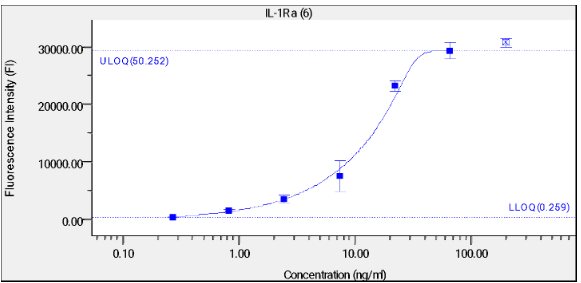
Titration curve with mean fluorescence intensity (MFI) against log α-IL-1Ra Abs concentration in μg/mL. Error bars represent standard deviations.
A screening of control sera is done:
In this, the analysis of the patient sera and control sera has been done. In this stage laser beam was passed through the sample mixture for conducting the screening of each antibody (Kurzrock et al. 2019). While passing through the colored beads of the multiplex sample the laser beam detects biotinylated bound IL-1B and IL-1Ra antibodies. Screenings enabled researcher to detect the magnitude of the PE signal which is directly proportional to the amount of biotinylated bound IL-1B and IL-1Ra. While conducting the screening process for detection and evaluation of the targeted antibodies in the Luminex sample, researcher needs to use the highly appropriate laser detection instrument. In this process, the researcher has used flex-map and Luminex 200 to apply the laser beam through the multiplex sample of biotinylated bound IL-1B and IL-1Ra to detect them.
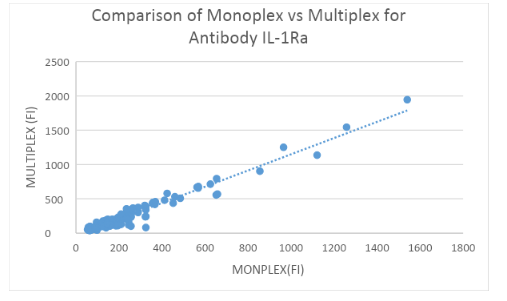
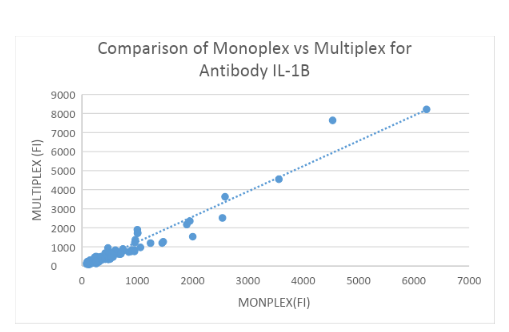
Measuring IL-1Ra and IL-1b Abs: 200 HC samples in Monoplex and Multiplex
Binding assays with IL-1Ra and IL-1b-coupled beads and α-hIgG Abs were performed to assess α-IL-1Ra Ab and α-IL-1b levels in controls both in Monoplex and Multiplex. Fluorescence was measured using the LUMINEX 200 System and plotted in figure 3A and 3B. No significant difference was found between the Monoplex and Multiplex for IL-1Ra(R = 0.954) and IL-1b (R = ??57) control group.
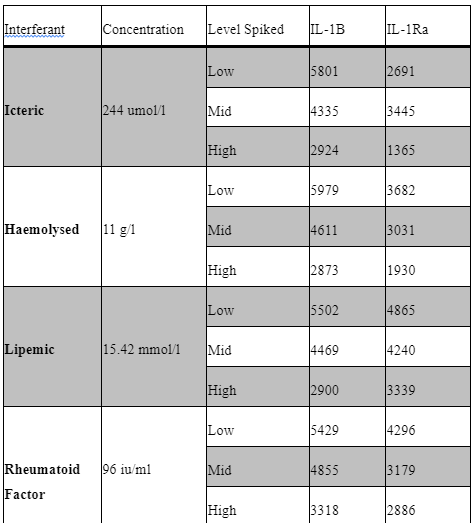
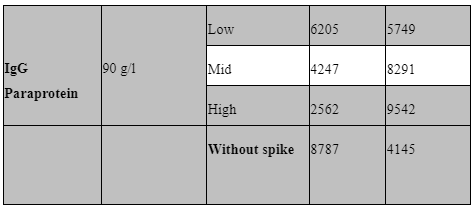
Interfering factors:
Potential interfering factors, including haemolysis, lipidaemia, hyperbilirubinemia, paraproteinemia, and high rheumatoid factor, were also evaluated to further define our sample acceptance criteria. Table 5 outlines the results of these studies. Briefly, samples with known levels of haemoglobin, bilirubin, paraprotein, rheumatoid factor and triglycerides were spiked into the low IQC at low (1:20), mid (1:10) and high (1:2) levels and then ran as standard in our assay. All samples used for interference testing were screened for anti-IL1B, IL-1R and IL-6.
Discussion:
The purpose of this project was to developing and validating a multiplex assay using Luminex technology to detect antibodies to IL-1Ra and IL-1b in patients with inflammatory disease. For this purpose, Il-1Ra and IL-1 b were successfully couples. Also, beads coupled to BSA and LPS were included as internal controls to identify individual with high levels of anti-BSA antibodies, leading to false-positive results, or individual with missing antibodies to LPS which leading to false-negative results.
Successfully coupled IL-1Ra and IL-1b and control beads BSA and LPS were tested in both Monolex and Multiplex with 200 healthy control samples. The mean serum IL-Ra concentration of healthy controls was approximately 250 pg/mL and mean serum IL-1b concentration of healthy controls was approximately 535 pg/mL. Also, not significant difference was found between monoplex and Multiplex in control samples.
Common interfering substances (i.e. hyperbilirubinemia, haemolysis, lipidaemia, rheumatoid factor and IgG paraproteinemia) were tested for sample screening to avoid potential false positive result. Samples with high levels of these substances need to be spiked with a known analyte concentration (Andreasson et al., 2015). No significant interference was seen in the haemoglobin, rheumatoid factor, Lipemic and bilirubin spiked samples for Il-1b and IL-1Ra. unfortunately, the paraprotein sample used appeared to have background positivity for IL-1Ra only.

Next Step:
Control sera will be used to stablish performance: Sensitivity, Specifitty and Inter/Intra assay precision. Also, patient sera will be analysed in Luminex for anti-IL-1Ra and IL-1B antibodies. Furthermore, patient samples will be tested functionally in IL-1Ra and IL-1b signalling by cytokine activation using PBMCs and STAT3 phosphorylation.

Continue your journey with our comprehensive guide to Understanding Coronary Heart Disease.
Reference list:
Al-Eitan, L.N., Al-Ahmad, B.H. and Almomani, F.A., 2020. The Association of IL-1 and HRAS Gene Polymorphisms with Breast Cancer Susceptibility in a Jordanian Population of Arab Descent: A Genotype–Phenotype Study. Cancers, 12(2), p.283.
Boldrup, L., Coates, P., Gu, X., Wang, L., Fåhraeus, R., Wilms, T., Sgaramella, N. and Nylander, K., 2021. Low potential of circulating interleukin 1 receptor antagonist as a prediction marker for squamous cell carcinoma of the head and neck. Journal of Oral Pathology & Medicine.
Dandona, P., Ghanim, H., Abuaysheh, S., Green, K., Dhindsa, S., Makdissi, A., Batra, M., Kuhadiya, N.D. and Chaudhuri, A., 2018. Exenatide increases IL-1RA concentration and induces Nrf-2‒Keap-1‒regulated antioxidant enzymes: relevance to β-cell function. The Journal of Clinical Endocrinology & Metabolism, 103(3), pp.1180-1187.
Dosh, R.H., Jordan-Mahy, N., Sammon, C. and Le Maitre, C., 2019. Interleukin 1 is a key driver of inflammatory bowel disease-demonstration in a murine IL-1Ra knockout model. Oncotarget, 10(37), p.3559.
Farooq, N., Chuan, B., Mahmud, H., El Khoudary, S.R., Nouraie, S.M., Evankovich, J., Yang, L., Dunlap, D., Bain, W., Kitsios, G. and Zhang, Y., 2021. Association of the systemic host immune response with acute hyperglycemia in mechanically ventilated septic patients. PloS one, 16(3), p.e0248853.
Grauers Wiktorin, H., Aydin, E., Christenson, K., Issdisai, N., Thorén, F.B., Hellstrand, K. and Martner, A., 2021. Impact of IL-1β and the IL-1R antagonist on relapse risk and survival in AML patients undergoing immunotherapy for remission maintenance. Oncoimmunology, 10(1), p.1944538.
Hajizadeh, Y.S., Emami, E., Nottagh, M., Amini, Z., Maroufi, N.F., Azimian, S.H. and Isazadeh, A., 2017. Effects of interleukin-1 receptor antagonist (IL-1Ra) gene 86 bp VNTR polymorphism on recurrent pregnancy loss: a case-control study. Hormone molecular biology and clinical investigation, 30(3).
Kurzrock, R., Hickish, T., Wyrwicz, L., Saunders, M., Wu, Q., Stecher, M., Mohanty, P., Dinarello, C.A. and Simard, J., 2019. Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1α antibody, in a phase III randomized study of advanced colorectal cancer. Oncoimmunology, 8(3), p.1551651.
Mendonça, L.O., Grossi, A., Caroli, F., de Oliveira, R.A., Kalil, J., Castro, F.F.M., Pontillo, A., Ceccherini, I., Barros, M.A.M.T. and Gattorno, M., 2020. A case report of a novel compound heterozygous mutation in a Brazilian patient with deficiency of Interleukin-1 receptor antagonist (DIRA). Pediatric Rheumatology, 18(1), pp.1-5.
Mortensen, S.B., Hansen, A.B.E., Mogensen, T.H., Jakobsen, M.A., Beck, H.C., Harvald, E.B., Lambertsen, K.L., Johansen, I.S. and Andersen, D.C., 2021. PYRIN inflammasome activation abrogates IL1Ra expression providing a new mechanism underlying FMF pathogenesis. Arthritis & Rheumatology.
Namai, F., Shigemori, S., Ogita, T., Sato, T. and Shimosato, T., 2020. Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Experimental & Molecular Medicine, 52(9), pp.1627-1636.
Pantalon, A., Obadă, O., Constantinescu, D., Feraru, C. and Chiseliţă, D., 2019. Inflammatory model in patients with primary open angle glaucoma and diabetes. International journal of ophthalmology, 12(5), p.795.
Parker, H., Ellison, S.M., Holley, R.J., O'Leary, C., Liao, A., Asadi, J., Glover, E., Ghosh, A., Jones, S., Wilkinson, F.L. and Brough, D., 2020. Haematopoietic stem cell gene therapy with IL‐1Ra rescues cognitive loss in mucopolysaccharidosis IIIA. EMBO molecular medicine, 12(3), p.e11185.
Porritt, R.A., Markman, J.L., Maruyama, D., Kocaturk, B., Chen, S., Lehman, T.J., Lee, Y., Fishbein, M.C., Noval Rivas, M. and Arditi, M., 2020. Interleukin-1 Beta–Mediated Sex Differences in Kawasaki Disease Vasculitis Development and Response to Treatment. Arteriosclerosis, thrombosis, and vascular biology, 40(3), pp.802-818.
Robuffo, I., Toniato, E., Tettamanti, L., Mastrangelo, F., Ronconi, G., Frydas, I., Caraffa, A., Kritas, S.K. and Conti, P., 2017. Mast cell in innate immunity mediated by proinflammatory and antiinflammatory IL-1 family members. J Biol Regul Homeost Agents, 31(4), pp.837-42.
Smith, C.J., Hulme, S., Vail, A., Heal, C., Parry-Jones, A.R., Scarth, S., Hopkins, K., Hoadley, M., Allan, S.M., Rothwell, N.J. and Hopkins, S.J., 2018. SCIL-STROKE (subcutaneous interleukin-1 receptor antagonist in ischemic stroke) a randomized controlled phase 2 trial. Stroke, 49(5), pp.1210-1216.
Yadav, D.K., Tripathi, A.K., Gupta, D., Shukla, S., Singh, A.K., Kumar, A., Agarwal, J. and Prasad, K.N., 2017. Interleukin-1B (IL-1B-31 and IL-1B-511) and interleukin-1 receptor antagonist (IL-1Ra) gene polymorphisms in primary immune thrombocytopenia. Blood research, 52(4), pp.264-269.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



