Improving Preterm Birth Outcomes
Introduction:
While we cannot save all the babiesborn too early or preterm (infants born before full 37 weeks gestation), we can improve outcomes because we now have the tools and knowledge,from decades of research on effective interventions that may save the lives of these vulnerable infants. Global estimates suggest that around 15 million babies are born too early worldwide each year (UNICEF, (2017)). More than 95% of these preterm births occur in LMICs (WHO | Preterm birth, 2017), with South Asia and sub-Sahara Africa contributing 60% of the total(Sankar et al., 2016).Of these, almost half die shortly after birth, making prematurity the leading killer of children under five worldwide.According to WHO (2017), 99% of all newborn deaths occur in LMICs with prematurity-related conditions accounting for over a third of all neonatal deaths.The survival rates contrasts starkly with high income countries(HICs) where nearly all preterm babies survive(Cooper, 2014). Despite this inequality in survival gap, much of the research focus on improving health outcomes of preterm infants, is in rich countries, and so, the progress to bridging this gap is slow(Franzen et al., 2017).Since prematurity related complications is a major contributing cause of death in the newborn period, greater emphasis should be on prevention of those complications(Lin et al., 2014). In the developing world, the progress to reducing neonatal mortality (deaths within the first 28 days of life) is very slow compared to HICs. It will “take a century for a baby born in Africa to have the same chances of survival as one born in a HIC”(Every Newborn, 2017). Mortality rates are decreasing by 1% per year in Africa, and for some LMICs like Pakistan, Central Africa and Afghanistan the pace lags far behind rest of the world.The global Every Newborn Action Plan(ENAP) set boldtargets toachieve by 2035and called for reducing neonatal mortality to fewer than 10 deaths per 1000 live births(UNICEF, (2017)).The target though ambitious, is possible. There is work in progress and overall global neonatal mortality rates are decreasing.
Causes of preterm deaths in LMICs
The three main killers of newborn infants are complications related to prematurity, intrapartum events and sepsis(Katz, 2018) .Of the 1.1million world’s preterm deaths (WHO | Preterm birth, 2017) each year, 75% are largely due to preventable causes, and lack of access to quality care, prevalence of risk factors and poor maternal health, are well-known underlying attributable factors common to most LMICs (Lawn, 2009). Preterm infants are at highest risk of dying due to immature organ systems particularly the immune system that is unprepared for the environment of extrauterine life. Although many succumb primarily to infections, other life-threatening conditions like necrotizing Enterocolitis (NEC) is associated with significant morbidity and mortality. Survival trends have been improving from essential care early in the first days of life such as newborn resuscitation and support measures for respiratory conditions related complications, yet NEC prevalence is increasing and remains a greater scourge in all countries(Berrington et al., 2012).
Why focus on NEC
To date, there is no known effective treatment for NEC, and prevention and early diagnosis is essential in ensuring chance of survival from this aggressive disease. Diagnosis can be challenging in setups where there is lack of expertise and radiological services, and because NEC is almost indistinguishable from sepsis,diagnosis of NEC is often missed, with ominous progression to severe stage, and death. With improving survival beyond the first weeks of life from advances in new born care like resuscitation and support care for respiratory conditions, NEC prevalence is likely to increase. A population based survey conducted over two decades from 1988-2008 in the United Kingdom reported a rise in deaths due to infection and NEC(Berrington et al., 2012). I will briefly review the epidemiology, clinical features, pathogenesis and prevention of NEC.
Epidemiology
There is paucity of reliable data on the true incidence of NEC internationally. According to a SR conducted in HICs, the reported incidences were highly variable across different settings mainly due to inconsistencies in definitions, and the criteria used to diagnose NEC. Incidences among infants 1500g ranged from 2% in Japan to 7% in Korea and Spain, with mortality rates in the range of 22% to 38%(Zani and Pierro, 2015). According to large and multicentred studies in Europe, Australia, New Zealand and North America, the estimated NEC incidence was around 13% (Nino, 2016). The global mortality rates are around 20-40%(Sharma and Hudak, 2013), 20-30% in HICs (Neu and Walker, 2011) with rates as high as 50% for those needing surgical treatment even in advanced care. The theory is, it maybe even higher in LMICs. In a retrospective review in Johannesburg(Ballot, Chirwa and Cooper, 2010) NEC was reported as only second to extreme prematurity as the leading cause of death in the very preterm babies. Despite its devastating natural history, associated mortality, and the frequency of occurrence in the most vulnerable group of neonates, we still don’t know how to prevent it. This premise holds true for LMICs(Eaton, Rees and Hall, 2017)
Description of NEC
NEC is an inflammatory bowel condition. It afflicts predominantly preterm infants, and putatively the most challenging gastrointestinal disease of prematurity worldwide. The disease process is characterized by inflammation with a variable degree of mucosal necrosis of the bowel(Huda et al., 2014). Symptoms are insidious, and signs are almost indistinguishable from neonatal sepsis, subtle at the onset, then rapidly progresses to fulminant gastrointestinal signs over a few days, terminating in signs of intestinal perforation, multi-organ dysfunction and shock(Kim, 2018).A typical patient is a preterm neonate who was stable and thriving. Suddenly, in the second week of life, he/she develops feeding intolerance, manifested by increasing gastric residuals, vomiting, and abdominal distension. The patient becomes noticeably lethargic, has recurrent apnoea, and appear pale or poorly perfused(Sharma and Hudak, 2013). The prognosis is terminal once abdominal signs such as oedema and abdominal discoloration appear. Surgery is an option in HICs but even with specialized care, the outcome is usually poor in cases involving severe NEC (Gephart et al., 2012) Diagnosis of NEC is challenging as there are variable definitions of NEC across the globe. The internationally accepted Modified Bell’s criteria commonly used,stages the disease on both clinical and radiological features(refer Appendix 1). Many studies have used this criterion to define NEC(Battersby et al., 2018). It has three stages. Stage 1 or suspected NEC includes nonspecific symptoms and signs such as temperature instability, apnoea, and lethargy; Stage 2 or Definite NEC include stage 1 plus metabolic acidosis, reduced platelets and radiological signs such as dilated loop bowels, Ileus, Pneumatosis Intestinalis; Stage 3 is severe or advanced NEC which include signs of intestinal perforation and Cardiorespiratory compromise or NEC requiring surgery.
What is NEC and what is not in LMICs
The characteristics of NEC are not well understood in LMICs. Data on morbidity and mortality are scarce. In HICs however, NEC accounts for 10-30% of deaths in very low birth weight infants (VLBW), defined as those weighing less than 1500g, and 35-50% in extreme low birth infants (ELBW), defined as infants weighing less than 1000g(Carter and Holditch-Davis, 2008).Often diagnosis of NEC is difficult when made clinically. Symptoms and signs like lethargy, Tachypnoea of respiratory distress syndrome or abdominal distension of spontaneous intestinal perforation share NEC-like symptoms thus, the true scope of the problem is unknown. NEC is also not commonly reported as a cause of death in many LMICs. Inadequate information from verbal autopsies and unreported deaths may contribute to the deficiencies and variable quality of the causes of death in some settings(Thatte et al., 2008). It is for this reason that NEC is somewhat perceived as a disease of HICs. Furthermore, sepsis could be overly implicated as the cause of death or morbidity in most circumstances. With more preterm deliveries occurring in LMICs, (Blencowe,2013) the frequency of NEC is possibly higher than is probably known, and likely a silent killer in LMICs. Pathogenesisand factors associated with NEC Despite extensive research on NEC, the pathogenesis remains elusive, however, plausible theories have been described. Briefly, the interaction between an underdeveloped immune defence system, an immature gut and nutrition has been described in the development of NEC. The presence of enteral feeds could either facilitate gut adaptation or cause microbial dysbiosis causing pathogenic colonization and disease(Siggers et al., 2011).Other factors commonly associated with NEC are further described below.
Prematurity
The most significant underlying risk factor for NEC is prematurity. NEC vulnerability is directly related to the gestational age. The more preterm the infant the higher the risk for NEC(Gordon et al., 2012). An immature gut has reduced gastrointestinal functions such as motility, digestion and absorption that predispose them to risk of mucosal injury(Tanner et al., 2015). Exposure to certain agents such as antibiotics or formula feeding leads to a cascade of inflammatory response causing intestinal injury and the development of NEC(Choi, 2014). This could explain why enteral nutrition, in the presence of an underdeveloped gut provides a substrate for colonization of pathogens. This perceived association may have propagated the practice of delaying feeds and using conservative feeding regimens for fear of NEC. Published studies however, have not shown any evidence to this effect and thus this debate continues (Tewari et al.; E, 2014)
2. Enteral feeding
Enteral feeding with formula is considered a common risk factor for NEC. Almost all reported cases of NEC occur after starting feeds(Huda et al., 2014). The association is unclear but the volumes and rate of advancing feeds, and high osmolality formula feeding have been implicated as risk factors(Caplan, 2018). Formula milk may facilitate the development of NEC as it does not share the same the protective factors such as Immunoglobulin and certain proteins, as human milk.
3. Tissue hypoxia
Hypoxia-ischemic conditions have also been linked to NEC. Hypoxic insults impair gut perfusion and consequently wall integrity leading to NEC(Shulhan et al., 2017). An observational study discovered NEC in 10% of late preterms most of whom had underlying risk factors such asphyxia, were SGA, or had a history of a congenital heart disease(Al Tawil et al., 2013).The mechanism of how these incite disease is not well understood.
4.Infection
Systemic infections have not shown to be a feature of NEC. However, the presence of radiological features of gas in the intestinal walls suggests bacterial fermentation as part of the disease process(Bell’s criteria). No pathogen has been isolated to date.
Interventions for prevention
Several strategies to prevent NEC have been tried but failed to prove efficacy. A recent Cochrane review on the effects of glutamine supplements, an essential amino acid, did not confer any positive impact on reducing NEC or mortality in preterm infants(Moe-Byrne, Brown and McGuire, 2016). Similarly, oral immunoglobulin has also been trialled to assess its immune-protective effects on the intestinal mucosa of preterm infants. The findings of this systematic reported that oral immunoglobulin did not decrease the incidence of NEC(Foster and Cole, 2001).The current interventions recommended by NEC guideline teams(Gephart et al., 2012)include antenatal Glucocorticoids, standardized feeding regimen, human breast milk feeding, Lactoferrin supplementation and Probiotics based on trials conducted in HICs. These interventions may be cost-effective and feasible, but there are marked differences in the risks and benefits when generalizing proven evidences to LMICs. Several factors may limit their effectiveness in these settings. For instance, HICs have round-the-clock monitoring, low risk of infection, good maternal health care, and resources and expertise are readily available compared to LMICs where these aspects of healthcare are limited. In addition, environmental factors in neonatal facilities such as inadequate infection control practices and different gut Microbiomes can also influence the response to the interventions. In view of this, this project seeks to determine the evidence base for preventative interventions for preterm infants in LMICs.

How the interventions might work.
The following are interventions that will be explored for effects on reducing NEC and the description of how each might work. These specific interventions may be feasible in LMICs in terms of level of care and resources they would require, and therefore relevant for practice in LMICs. There is convincing evidence that antenatal steroids may reduce adverse outcomes in prematurity including NEC. Glucocorticoids work by accelerating organ system maturation including the gut and immune system. Several RCTs have demonstrated reduced incidence of respiratory distress syndrome and neonatal death in preterm neonates, however there is insufficient information on its influence on NEC(Gephart et al., 2012). Feeding practices in preterm infants were once based on anecdotal experiences where clinicians used variable regimens. Now, several studies suggest standardizing feeding protocols achieves more beneficial outcomes. The elements of a protocol could include when to start and advance feeds, rates of increments, frequency of feed (continuous or intermittent feeding), types of feeding and supplements to feeds. The literature offers some reasons why adhering to a set feeding regimen might benefit preterm infants. A SR of observational studies reported a reduction in risk of NEC by almost 87% in LBW (95%CI 0.03, 0.50)(Patole and de Klerk, 2005).The same group also demonstrated positive results on reduction of NEC in a SR of “before” and “after” studies(Jasani and Patole, 2017).Similarly, another group found that in addition to reduction in NEC, other important outcomes were significantly reduced such as improved growth outcomes, fewer days to attaining full feeds, decreased incidence of sepsis, and reduced length of stay(McCallie et al., 2011).Despite the positive findings across studies, data exist where the results are unfavourable including risk of NEC with feedings. The capability of generalisation to different populations is concerns because effects from these trials may not be reflected in other settings particularly where monitoring and adherence to protocols are not practised. Moreover, there are still inconsistencies in the actual feeding regimes. The ongoing debate on continuous nasogastric versus intermittent bolus feeding is an example where a RCT showed more feeding intolerance with the latter feeding strategy (Dsilna et al., 2005). It is established that human breast milk is protective against NEC(Patel, 2017). Breast milk given as the first feed establishes “good” bacteria that promotes a non-pathogenic microbiota and enhances gut maturation (Bergmann et al., 2014). It contains a host of protective factors vital for a preterm infant. These include Prebiotic Oligosaccharides that stimulate colonization of the intestinal flora inducing gut maturation; Immunoglobulin, and enzymes that influence immunity and mucosal protection from pathogens(Meinzen-Derr et al., 2009).When mother’s milk is not available or insufficient, the alternatives are donor milk(DM) or formula. WHO recommends pasteurized DM as the next option, and formula as the third and fortification, when required for preterm feeding(WHO, 2018).Pasteurization is costly and in some setting, not be feasible, or acceptable for various cultural, personal or religious reasons(Coutsoudis, Petrites and Coutsoudis, 2011). In addition, while pasteurization safeguards the infant from transmissible infections, the process has disadvantages. Heating denatures protein in breast milk reducing effectiveness of its immune-protective properties on the gut, as well as the nutritive benefits. Despite this drawback, there is evidence of its advantage over formula in reducing the incidence of NEC(Kantorowska et al., 2016). Currently, in many HICs, DM and even mother’s milk is fortified to supplement extra protein and energy in preterm infants receiving small volumes of milk. Concerns with fortification are the potential to increase osmolality. The high osmolality causes slow gastric emptying leading to feeding intolerance or possibly NEC(Kreissl et al., 2013). Formula milk on the other hand is easily available and offers nutrients for growth and development for the growing infant but does not provide the immune-protective components contained in breast milk(Lok et al., 2017). It is also not well understood why even exclusively breastfed infants still develop NEC. Lactoferrin is a protein found in breast milk. Concentration is highest in colostrum, andin milk of mothers who delivered preterm(Albenzio et al., 2016).It has antimicrobial propertiesthat enhances gut immune development in the new born. Lactoferrin acts with Lysozyme and other antimicrobial proteins in breast milk to prevent invasion by destroying pathogens, and eliminate toxin effects on the intestinal mucosa(Sherman, 2013).Lactoferrin has shown to act synergistically with probiotics to reduce sepsis(Manzoni et al., 2009).A RCT evaluating the effects of bovine Lactoferrin formula on morbidity, found a reduced incidence of respiratory and diarrheal-related diseases in infants(Chen et al., 2016). Previous trials have also shown no correlation between levels of LF and mother’s nutritional status so even if mothers are undernourished the LF levels are unchanged.
The role of Probiotics in prevention of NEC has been exhaustively researched, and despite a replica of statistically significant outcomes from clinical trials, there remains several uncertainties to its use universally(Chang et al., 2017). Probiotics work therapeutically by colonizing the preterm GI tract with non-pathogenic organisms. They improve motility, mucous quality and alter the balance in the intestinal microbiome limiting overgrowth of pathogens in favour of nonpathogens thus reducing the development of NEC(Hunter et al., 2008). To date, no established probiotic strain has been recommended though routine probiotics used in many neonatal intensive care units are bifidobacterium bifidum, and lactobacillus acidophilus either singly or in combination(Samuels et al., 2016).Several RCTs have shown efficacy of Probiotics in reducing NEC(Braga et al., 2011) yet there are caveats concerning varying results regarding strain type. Different preparations produce different outcomes with efficacy in some and not others.
Why was it important to do this review?
NEC is associated with significant morbidity and mortality in preterm infants, and surgery for severe NEC may not be an option in LMICs. The mortality with surgery even in HICs is 30-50%(Zani and Pierro, 2015), and long-term outcomes with short bowel syndromes is poor. Currently, there is no effective treatment for NEC and prevention strategies are needed to improve survival from this condition. With the increasing numbers of preterm infants and improving survival through care in the first few days of life, these infants are at risk of developing NEC. Identifying potential effective interventions that are pragmatic for LMICs should therefore be given greater attention. Given the overwhelming literature, and research on the prevention of NEC conducted mainly in HICs, this review was important to bring together the existing evidenceon multiple interventions from SRs in one review, to assess their relevance for practice in LMICs. The review will offer an accessible collated summary of evidence for clinicians in these settings to evaluate and use to inform practice, assist decisions by policy makers and support development of clinical guidelines in LMICs. I have not found any existing review of SRs on this topic. The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analysis) statement or checklist for reporting systematic reviews was used as a guide for this review to ensure transparent and systematic reporting of how well the review was conducted. A robust methodology and accurate interpretation of results can ensure reproducibility of the findings, reliable and credible results that end-users could support for implementation into clinical practice(Liberati et al., 2009). It also allows them to evaluate the strengths and limitations of the review. PRISMA checklist gives guidance on reporting recommendations for an evidence-based quality review from the title to conclusion section. As a new researcher, PRISMA served as a guidance method on the requirements I needed to follow to ensure my reporting of the whole review and more importantly, the methods and findings were clear.
AIM:
This review aims to assess the evidence for interventions for the prevention of NEC in preterm infants in LMICs
OBJECTIVES:
To provide an overview of NEC and the preventive interventions relevant to LMICs.
To identify SRs of randomized and quasi-randomized controlled trials and appraise the quality of the evidence of each intervention.
To provide a narrative synthesis of the evidence and discuss the pragmatic interventions relevant to LMICs.
To discuss the implications for practice in LMICs to prevent NEC in preterm infants.
The review will answer the following clinical questions:
What are the effects of each intervention on the risk of developing NEC in preterm infants (less than 37 weeks gestation) in LMICs?
Which interventions would be optimal in preventing NEC in preterm infants in LMICs?
Research Question
Several interventions have been defined, and the available evidence suggests a positive impact on the incidence of NEC. How can these potential effects of the interventions be applied to preterm infants in LMICs where most preterm births occur, with differences in risk factors and neonatal care settings?
2.METHODODOLGY
In this chapter the methodology is discussed in detail to achieve objective 2.
2.1 Selection criteria
Types of studies
This review included only SRs of randomised controlled trials and quasi-RCTs that addressed the research questions and reported NEC as an outcome. The focus of this review was to evaluate the evidence for multiple interventions that may be relevant to LMICs hence rather than repeating searches on RCTs already done by reviewers of SRs, I chose to evaluate SRs. Conducting a separate SR with meta-analysis of RCTs for each intervention would be too ambitious in the short time given for this project. SRs are reviews that systematically appraise and synthesise the evidence of primary studies to answer specific research questions using a set predefined methodology. This differentiates SRs from other types of reviews such as literature, narrative and expert reviews that were excluded in this review. RCTs were selected over observational studies to avoid confounding factors thereby ensuring reliable and high standard of evidence for evaluation of effectiveness of the interventions. The criteria used to select SRs was based on the population, intervention, comparator and outcome format(PICO) (refer Box 1).All the reviews that did not meet the inclusion criteria were excluded from the review including SRs with observational studies, RCTs and other descriptive studies.

The PICO format was applied as inclusion criteria for selecting the SRs:
Types of participants
The population of interest were preterm infants, defined as infants less than 37 weeks gestation. The selection excluded SRs limited to preterm infants less than 25 weeks gestation because survival of this preterm cohort in LMICs is poor. Moreover, viability for preterm infants in LMIC is >28 weeks gestation(Lawn et al., 2011). The SRs include late preterm(32-37 weeks),very preterm (28-32 weeks), and extremely preterm(28 weeks)(WHO | Preterm birth, 2017).
Types of interventions and comparators
Five pre-specified interventions were considered based on preliminary literature review of all the interventions on prevention of NEC. These are current strategies that may prove to be pragmatic and cost-effective for LMICs. Antenatal steroids are currently standard protocol for preterm labour less than in many countries including LMICs
Breastfeeding is the preferred feeding in LMICs, and pProbiotics are relatively affordable. Lactoferrin supplements according to the literature are inexpensive even for LMICs(Turin et al., 2014). The interventions are:
Antenatal steroids (dexamethasone or betamethasone) versus placebo or no steroids.
Feeding regimens (include timing of feeding, rates, volume of maximum feeds, methods of feeding) versus standard practice.
Human breast milk as the standard type of milk for enteral feeds versus formula milk or mixed feeding.
Oral Lactoferrin supplementation given with enteral milk feeds versus placebo or no Lactoferrin.
Probiotics versus Placebo or no Probiotics.
Types of outcome measures
This review was streamlined to focus solely on the occurrence of NEC either as a primary or secondary outcome as the research question focuses exclusively on NEC, but because mortality is an important outcome for any intervention, data on mortality was also included.
NEC should not be isolated from these important clinical endpoints especially when considering which interventions to adopt for LMICs. This information can support feeding policies and practices in these settings.
Feed intolerance (FI) was also chosen as an outcome. This is important as this reflects the objectives of the review, and the relevance to the main outcome of NEC. FI maybe closely associated with risks of NEC and is often recognized as initial or early manifestations of NEC in low income settings where radiological resources or the expertise may not be available to aid in making diagnosis.
NEC was staged as any, suspected or confirmed using the modified Bell’s criteria (see Appendix 1). The SRs defined confirmed NEC as diagnosis by at least two of the following features: Abdominal radiograph showing pneumatosis intestinal is or gas in the portal venous system or free air in the abdomen; abdominal distension with x-rays demonstrating gaseous distension or frothy appearance of bowel lumen (or both); blood in stool; lethargy, hypotonia, or apnoea (or combination of these); or a diagnosis confirmed at surgery or autopsy.
Study setting: Any location.
The review selected SRs that included all countries for consideration of relevancy to LMICs. LMICs is defined as Gross National Income(GNI) per capita between $996-$3895. HICs defined as GNI per capita of $12,056 or more (World Bank, 2018).
2.2 Search strategy.The search strategy commencedon the 18th May 2018. A comprehensive search was carried out in all relevant databases, and grey literature to ensure no study was missed. EBSCO Discovery was used to initiate the search, and to scope the topic for all potential studies. Specific databases for systematic reviews were then searched to narrow the search using limit indexes. I performed an extensive search to capture all studies in the databases that are listed below.
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
Medline
Web of Science
CINAHL
GLOBAL health
Google Scholar
LILAC
Trip database, International initiative EPPI Centre, Campbell Collaboration, Joanna Briggs.
Online search via Google
Ongoing SRs in PROSPERO were also examined, as well as abstracts of conference proceedings from professional organization such as the Pediatric Academic Society(PAS) and Perinatal Society of Australia and New Zealand(PSANZ). The reference lists of all the included reviews were also manually searched for potential reviews. The internet was also searched for supplementary information. Articles that were not available in the LSTM library were retrieved by interlibrary-loan and via requests to authors of the SRs.
2.3 Search methods
The PICO search strategy was used to identify key terms from the research question. A list of synonyms was compiled for each key word before the search. Controlled terms (MeSH terms) were identified for key words from database indexers and citations of relevant studies. A test search was then carried out using the mentioned criteria, in developing countries. This preliminary search was done to evaluate my search skills and to see if the strategy was adequate to generate results. The search produced insufficient results when LMICs was included so professional help was sought for assistance. The librarian also provided information of other databases specific to systematic reviews.
2.4 Search term combination for the electronic databases.
The full search string of controlled terms, free texts (see Box 2), and their combination with their Boolean operators “AND”, “OR” are included in Appendix 2. A similar search strategy was adapted for all databases. The search was limited to review/systematic review/birth to 23 months/human/ years 1940 to present. Only studies in the English language were selected due to lack of access to translation services and funds to pay for the translation.


2.5Selection of reviews
The PRISMA flow chart describes the processes used to identify relevant literature. Each electronic database was searched separately for each intervention. Records were saved on Endnote in separate folders, and the results were later combined.
The selection process was carried out in the following steps:
Identify records for relevance using the inclusion criteria
Deduplication of the identified records (done manually)
Screen all titles and abstracts of the selected records for eligibility.
Retrieve and read full text articles of the potential relevant records
Save and record reasons for exclusion on forms designed for the search.
Report results of the included articles for synthesis (Using PRISMA diagram)
Appendix 4 lists the excluded articles with reasons for exclusions. To ensure potential articles were not missed, the screening was done three times and if the decision was unclear if the article was eligible, the full-text article was retrieved to be read. This exercise was a means of reducing bias in the selection of the reviews as there was just one reviewer.
2.6 Data extraction and management
Two data extraction forms were designed based on the information I needed for both the descriptive analysis and the analytical analysis. One was purposed to enter the characteristics of the SRs, and the other for the outcome estimates for the interventions. A pilot exercise was done with the first two reviews to assess if the extraction form captured all the necessary data. Changes to the form were then made before continuing data collection. Data from each SR was then summarized and presented in tables, grouped under each specific intervention.
The outcome estimates for NEC, mortality and feed intolerance from the summary tables and forest plots of the SRs were collected and summarized in a table. The interpretations of the results were also included.
2.7 Data synthesis
Information on the characteristics of included SRs was extracted and summarized in a table. Data collected included the title and first author, year of publication, study design, number of trials, number of infants, review objectives, population, study setting and summary of risk of bias of the primary studies (see Table 1).This “characteristic of included SR” table provided a synthesis of information that compared interventions from different reviews, evaluated qualities of the primary studies, and importantly features that could support decisions on generalisation to LMICs. This was done to assist in meeting objective 3. Effect estimates, presented as relative risks (RR) with the 95% confidence (CI) intervals were also summarized and tabulated (refer Table 3).A narrative synthesis of the results is discussed in the ‘Results’ section. The data was solely extracted by myself.
2.8 Quality Assessment of included reviews
This process was done after data extraction and analysis of the results. I thought this was important to avoid reporting quality bias thus all the SRs that met the inclusion criteria were included irrespective of the quality. The Assessment of Multiple Systematic Reviews (AMSTAR) 2 tool was employed to appraise the methodological quality of included SRs. This tool is a revised version of the original AMTSAR which was designed to appraise SRs that include only RCTs or non-RCTs on healthcare interventions. This current version consists of 16 questions requiring a “Yes”, “NO” or “partial Yes” response. Broadly, these include a comprehensive search, critical appraisals of the studies and the analysis of the results. The critical domains include questions 2, 4,7,9,11,13, and 15 (Appendix 3). A well conducted review should not have weaknesses(‘No’ response) in the mentioned critical domains. For this review, the methodological quality was assessed solely by the author and the results were tabulated (Table 2) with the rating of the overall degree of confidence in the results of the review. High confidence is awarded if there is no or one non-critical weakness; moderate confidence is having more than one non-critical weakness; low if there is one critical domain with or without non-critical weakness, and critically low confidence in the results is defined as having more than two critical weaknesses with or without non-critical domains. A summary text of the appraisal assessment table is provided in the Results section.
3.RESULTS:
This section gives an overview of the search results, characteristics of the included studies, the methodological quality appraisal of the SRs, and the results of interventions. The format is presented in that same order.
3.1Selection of Reviews
The search identified 106 eligible articles in total and generated 16 SRs for final analysis after examining full-text articles. The final selection was grouped into the five interventions. Of the 16, I found no SR for antenatal steroids, five for feeding regimen, 5 for human milk feeding, one for Lactoferrin and five for Probiotics. The search process was the same for each intervention. A systematic approach was done in the electronic databases such as Medline, Cochrane library, CINAHL, Global Health, and Web of Science, citations that appeared relevant were retrieved and saved. Additional sources of records obtained from other databases, grey literature, journals, and reference lists were combined with the initial search. After removing the duplicates, titles and abstracts of the remaining records were screened. The eligible records had full-text articles retrieved and assessed against the inclusion criteria. The search was completed on the 11th of June. Below are the flow charts and the summaries of the search results for each intervention. The excluded articles with justification for exclusions are detailed in Appendix 4.
3.2 Results of search
Antenatal Steroids:
A total of 119 records were obtained from the bibliographic databases. Nineteen more were identified through other sources. After discarding duplications, 72 titles and abstracts were screened for relevancy concerning the research question. Of those, 10 articles were eligible for full-text article assessments. Unfortunately, none of these met the inclusion criteria. The reasons for exclusion are recorded in the flow chart. A few SR included non RCTs and therefore were excluded. With an unproductive result, a re-run was done to reassess the 72 titles and abstracts if there were other articles that may have been missed. Results proved to be the same.

Feeding regimen
Records identified from electronic databases and manual search were 430 in number. From the 76 records identified after removal of duplication, 34 were eligible for full-text article assessments. Only five reviews were included for the final analysis. The reasons for the exclusions are provided in Figure 2 below.

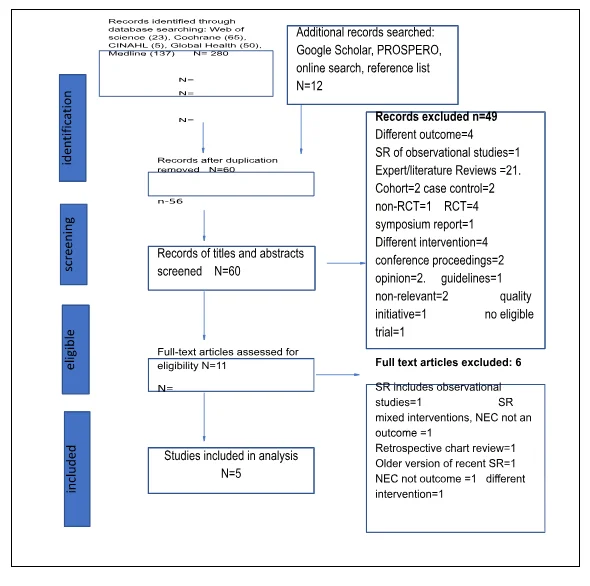
Human milk feeding
The search identified 292 records that appeared relevant in context to the research question regarding this intervention. After removal of duplicates, 60 remained. Following the screening of titles and abstracts, 11 were eligible for full-text article assessment. The final reviews that met the inclusion criteria was five (Figure 3). The articles excluded were mainly SR with observational studies, had different outcomes, and older versions of one of the included SR.
Lactoferrin
Only one SR was generated for analysis following the full-text assessment of the 17 potential articles. Excluded articles were mainly RCTs and literature or expert reviews. One SR did not report a meta-analysis of the primary studies and another SR examined Lactoferrin in colostrums but did not include NEC as an outcome.

Probiotics
The search was extensive as this is the most studied intervention on NEC prevention than any other. The search produced 477 records from the major databases and 45 more from additional sources. After removal of duplicates, 196 remained for screening. Forty-one articles were eligible following the screening process of titles and abstracts. The final number of inclusion for analysis after rescreening the eligible articles was five. The articles excluded were mainly expert reviews and meta-analysis.

3.3 General description of included SRs
The final selection totalled 16 SRs. Of the 16, half were Cochrane reviews and the rest were non-Cochrane. Six were updated versions of earlier reviews (Oddie,2017; Morgan, 2014; Morgan,2013;Quigley, 2018; Pammi, 2017). All except one (McGuire,2003), were published after 2010.Study designs included RCTs or quasi-RCTs or both. The number of trials ranged from one to 35, while the number of participants ranged from 64 to 7325 infants per review. Participants included in most reviews defined preterm infants by gestational age or based on birth weights, and the range was from ≤ 28 to ≤ 37 weeks or 1500 to 2500g. Five SRs addressed different feeding regimens; initiation of feeds, timing of progressive feeds, rate of advancement, maximum feed volumes and how to feed. The human milk feeding intervention included exclusive human milk feeding and milk fortification. All included SRs had NEC as diagnosis confirmed by clinical features, or at surgery or at autopsy. Whilst only two SRs had ‘any’ NEC, the rest specified NEC stage 2 or more by modified Bell’s classification (Appendix 1)as the outcome. NEC was the primary outcome in 14 SRs. The primary studies from the SRs were conducted mostly in middle and HICs except for one (Deshpande,2017) which only included trials from10 LMICs of different continents. The reviewer’s appraisal of the quality of the SRs based on the risk of bias (ROB) assessments is reported where available. Nine SRs reported quality rating, ranging from fair to good. Further information isprovided available in Table 1.






3.4 Quality Assessments of the SRs
The methodological quality of the included SRs was assessed and summarized in Table 2. A visual overview showed only 2 SRs were able to report the funding sources for the RCTs. Based on the appraisals (AMSTAR tool), eight reviews were judged to be of high quality. Four were interventions on feeding regimen, two from human milk feeding and two from Probiotics. These SRs exhibited robust methodology in selection of studies, critically appraising and analysing results of the trials but flawed on report funding sources of the trials. The only SR for Lactoferrin was assessed low quality (Pammi,2017). This review was limited because it did not investigate and discuss publication bias. Two older reviews on the human milk intervention (McGuire 2003, Ben X, 2012) were also of low quality due to failure to state if a protocol was done before review was undertaken. Similarly, two probiotic SRs (Deshpande, 2017; Thomas,2017)were limited for the same reasons. The others with moderate quality, (Premji, 2011), showed well-conducted analysis of the evidence but acquired limitations related to reporting funding sources, and study selection processes (Brown,2016). Rees et al,2017 showed multiple critical weakness and therefore judged to be of poor reliability to provide an accurate summary of the results from the available studies.

3.5 Results of the interventions
The results are described for each review under the four interventions. Summary of effect estimates are detailed in Table 3.
3.5.1Feeding regimen
There was no more than one review for each feeding strategy. All the five included were Cochrane reviews that described five distinct clinical scenarios of feeding strategies. These included the timing of introduction of feeds, rate of advancement of feeds, timing of introduction of progressive feeds, methods of feeding namely continuous versus bolus feeding, and the maximum volumes of milk feeds. The number of trials in each review ranged from 7-10 except for the study by Abiramalatha (2017) that had just one trial which included 64 infants. This study was done in India comparing high versus low volume feeds. Most included SRs had trials mainly from middle and high-income countries. The population studied in all the reviews were mostly infants who had been less than 1500g of weight or less than 32 weeks old. One study involved all infants less than 37 weeks or those who weighed less than 2500g (Abiramalatha,2017). All the reviews reported lack of blinding of the caregivers and personnel to the interventions, and outcome assessments which may have resulted in downgrading of the quality of the evidence for some reviews. None of the SRs on the feeding regimens showed any evidence of statistically significant effects on the risk of NEC incidence, feeding intolerance and mortality. The following details the outcome effects for each feeding strategy from the SRs (refer Table 3). Morgan et al(2013)included nine trials and 754 preterm infants comparing effects of trophic feeding to enteral fasting on the risk of NEC. The evidence provided showed that early trophic feeding did not have any statistical significant effect on the incidence of NEC (RR1.07, 95%CI of 0.67 to 1.70) or all-cause mortality (RR0.66, 95%CI 0.41,1.07). The review assessing the effects of delayed introduction of progressive feeds later than four to seven days after birth compared to less than four days included nine trials and 1106 infants. The effects on the incidence of NEC demonstrated a RR of 0.93 with a 95% Cl of 0.64 to 1.34. The evidence suggests that delaying the feeds beyond four days did not have statistical significant effect on the risk of NEC. The review comparing slow advancement of feed volumes at daily increments of 15-20ml/k/d (24ml/k/d)to faster rates of 30-40ml/k/d included 10 trials randomizing 3753 VLBW infants (Oddie 2017). The relative risk(RR)was 1.07 with confidence intervals(CI) of 0.83 to 1.39. Meta-analysis showed no evidence of a statistically significant effect on the risk of developing NEC (primary outcome). The results were similar for FI and mortality with no statistically significant difference between the two groups(RR 1.2, 95% CI: 0.95,1.50) and (RR 1.15, 95%CI: 0.93,1.42)respectively. The quality of the evidence was graded high but downgraded to moderate due to lack of blinding in all the trials. The only trial that assessed high volume feeds of >200m/k/d compared to standard volumes of 200ml/k/din 64 infants less than 37 weeks or weighed less than 2500g showed a RR of 1.03, 95% CI of 0.07 to 15.78. There was no significant difference in increase in the risk of NEC stage 2/3in infants given high volume feeds and standard feed volumes. The confidence interval of the effect estimate was very wide with the upper limit of more than 15-fold increase and evidence in the risk of bias downgraded the quality of evidence to very low. The seven trials that compared continuous nasogastric tube feeding to intermittent bolus feedings found no significant difference between the feeding methods (RR 1.53, 95%CI 0.41 to 1.07).
3.5.2 Human milk feeding
Quigley et al (2018) assessed formula milk compared to donor milk for feeding preterm infants in 8 trials enrolling 1605 infants. This review reported NEC as a secondary outcome. Meta-analysis showed the risk of developing NEC in the formula-fed group was about two times more than the human milk-fed group (RR 1.87, 95% CI 1.23 to 2.85). The data did not show any effect on all-cause mortality (RR1.11, 95%CI 0.81,1.53)The reported GRADE rating was of moderate quality evidence. The review (McGuire 2003) assessing the difference between DM and formula on NEC prevention included just four trials randomizing 343 preterm infants. The included trials were those conducted over 30 years ago. Individually, the trials did not show any significant difference in the incidence of NEC however, meta-analysis of the combined trials gave a borderline statistical difference (RR 0.34, 95%CI of 0.12 to 0.99). Similarly, confirmed NEC had a near statistical significant difference: RR of 0.25, 95% CI of 0.06 to 0.98) The review by Brown et al(2016) evaluated if addition of multi-nutrients to HM would improve neonatal outcome without increasing risk of NEC and feeding intolerance. Multi-nutrient fortification are liquids or powders that contain extra nutrients such as protein that are usually given to preterm babies with nutritional deficits(Brown et al., 2016). In this review, NEC was a secondary outcome. Meta-analysis of 11 trials which included 882 infants showed that HM fortification does not increase the risk of NEC incidence in preterm infants (RR 1.57, 95%CI 0.76 to 3.23) however this was of low quality evidence. The results were similar for subgroup analysis in VLBW (RR1.19 95% CI 0.49,2.88) and in the trials set in LMICs (RR1.73, 95%CI 0.33,9.11). Miller et al(2018) assessed the benefits of any human milk(donor or mother’s) concerning the risk of NEC (any stage) compared to formula milk in infants weighing 1500g or less. Six trials were included in this review. Two were observational studies and 4 were RCTs. The review pooled the two study designs separately but only the RCT group analysis is included herein. NEC was the primary outcome. These were the results of the meta-analysis of the four RCTs.
Exclusive HM versus exclusive formula: showed no significant difference in incidence of any NEC (RR0.17, 95%CI 0.02,1.32)low certainty);severe NEC (RR 0.09, 95% CI 0.01,1.64) low certainty
Any mixed human versus exclusive PTF: no trials
High versus low dose HM: Relative risk was 1.07(95% CI 0.89,2.0) Moderate evidence; For severe NEC(RR 0.95 CI 0.73,1.25)
Unpasteurized vs pasteurized human milk, only one trial reported any NEC or severe NEC. For both outcomes, there was no statistical significant difference between the two groups: any NEC (RR 0.71, 95%CI 0.43, 1.18). and severe NEC with a RR of 0.69 (95%CI 0.43,1.1)
3.5.3 Lactoferrin
This review included six RCTs that were published over the last three years. A total of 1,041 preterm infants were enrolled in this review. The trial settings included one low-income country among the seven countries (Kaur 2015). This review evaluated the effects of supplementing Lactoferrin to enteral feeds compared to no intervention/or placebo. NEC stages 2 or more was the primary outcome. Although the methodological quality of the included trials was good, the quality of body of evidence by GRADE assessment was low to due to risk of bias and moderate to severe heterogeneity in the meta-analysis. The four trials that reported NEC as the primary outcome included 750 infants. Meta-analysis showed a risk reduction of NEC by 60% (95%CI0.18,0.86) compared to the group without Lactoferrin supplements. The quality of evidence however was rated low due to significant heterogeneity and the lack of explicit randomization and allocation concealment in one study.
3.5.4 Probiotics
There were five SRs that evaluated the association between Probiotics use and NEC in preterm infants. The trials per review were large. The smallest enrolled 3875 participants(Rees et al., 2017), and the largest review had 7325 participants(Thomas et al., 2017). The first review identified 20 RCTs and included 5529 infants across both HIC and LMICs (Alfaleh,2017). It assessed effects and safety of any Probiotics given for four to six weeks, on prevention of NEC in preterm including ELBW. Eleven of the trials were classified as high-quality trials. Meta-analysis of the data showed statistically significant reduction of severe NEC (RR 0.43, 95%CI 0.33,0.56) in all infants less than 37 weeks gestation. Two studies did not show significant effects in ELBW infants (RR 0.76, 95 % CI 0.37,1.58). Similarly, positive significant benefits were shown for reducing NEC-related mortality (RR 0.0.39, 95%CI 0.18, 0.82). The review reported no adverse effects with Probiotic use. The second review (Aceti 2015), assessed specific Probiotic strains to prevent NEC in preterm infants. The review identified 26 trials involving 6605 infants.The RR was significantly lower in infants given Probiotics (RR 0.47, 95%CI 0.36,0.60). Very similar effect size was found in VLBW (RR 0.48, 95%CI 0.37,0.62) The third SR assessed safety and efficacy of Probiotics in reducing NEC and mortality in preterm infants 37weeks (Deshpande 2017). This review identified 23 RCTs conducted only in 10 LMICs. The reduction in incidence of NEC stage ≥2 was statistically significant (RR0.46, 95% CI 0.34,0.61). Mortality reduction was 27% (95% CI 0.59,0.90). Thomas et al (2017) was the largest SR. It included 23 RCTs enrolling 7325 VLBW infants (1500g) set in varied settings including LMICs. The review assessed the association between Probiotics and NEC stage ≥2, as well as NEC-related mortality in the mentioned preterm cohort. These were high-quality trials based on the reviewer’s report. Findings showed a statistically significant risk reduction of NEC (RR 0.57, 95%CI 0.43,0.74)but did not reach statistical difference on NEC-related mortality (RR0.64, 95%CI 0.38,1.07). Rees,(2017) assessed Probiotics and impact on surgical NEC (stage ≥ 3). Although the focus was on stage 3 NEC, they were able to meta-analyse the data from all trials for stage 2. These estimates showed a risk reduction of 36% in stage 2 but a non-significant effect on stage 3 (RR0.74, 95%CI 0.51, 1.05).Probiotic administration in ELBW feeding is not associated with decreased risk of NEC but also did not reach significant difference.




4. DISCUSSION
4.1 Discussion of results
This overview of SRs evaluated the effects of five specific interventions that may prevent NEC in preterm infants in LMICs. Overall 16 SRs were identified. The AMSTAR appraisal was not used for inclusion decision for this review. All SRs that met the inclusion criteria were included for this review. Eight SRs were assessed to be of high quality regarding reviews, two were moderate and the rest were judged to be low. The two high quality reviews in the HM feeding were the most recent SRs.
This section discusses the findings under each intervention with indications of the quality of evidence where available, the quality assessment of the review based on AMSTAR and recommendations.
Antenatal steroids
Dexamethasone or hydrocortisone is given as a single course for preterm labour mainly to prevent respiratory distress syndrome in preterm infants but its effects on NEC has not been clearly determined. Because this is a routine practice even in LMICs, I wanted to examine the evidence of the impact steroids have on NEC incidence. There were two SRs that met the criteria however the gestational ages (GA) of the participants were less than 25 weeks. Viability in LMICs is 28 weeks whereas HICs such as the UK, it is 24 weeks(BPAS, 2018),therefore these SRs were not included for analysis as they were not applicable to LMICs. Recommendation: RCTs set in LMICs are needed to evaluate this intervention as there are many factors such as rates of infection, inaccuracy of GA, delivery care that could confound its true effects on NEC in these settings.
Feeding regimen
Slow advancement on NEC:
The rate of incremental feeds may influence certain outcomes in preterm infants such as growth, feed tolerance, and importantly the risk of NEC.In this SR, slow advancement, defined as daily increments of 15-24ml/k, had no impact on NEC (RR1.07,95%CI: 0.83,1.39) compared to the fast advancement group (30-40ml/k/d) in preterm infants. This suggests that both feeding rates may be safe. This contrasts the findings in previous studies which showed that slow feeding advancement is associated with reduced risk of NEC(Patole and de Klerk, 2005). In another study(Rozé et al., 2017), risk of NEC was increased with slow feed rates. Despite these conflicting evidences, the findings of this review could be considered to have validity. The review was sufficiently rigorous in the design methodology, and the quality of evidence was moderate (downgraded from high because of unblinding which is unavoidable for this intervention). Thus, the findings may be generalisable to LMICs. More than half of the trials were conducted in LMICs and the population studied were stable infants not requiring specialized care, and small for gestational age(SGA) infants, a population that contribute the majority of LBW in LMICs(Lee et al., 2013). Rapid advancement feeds allow faster time to reach full feeds thereby minimising the prolonged hospital stays and its associated complications. The data also did not show an effect on feed intolerance (RR 1.2, 95%CI 0.95,1.50), and mortality (RR1.15,95% CI: 0.93,1.42).These are supplementary findings that can support its implementation in LMICs. Recommendation: Based on the evidence that rapid advancement of feeds in preterm is safe and the quality of the review is high, the author recommends this strategy and the evidence can support guidelines for preterm feeding in LMICs.
Effect of delayed introduction of progressive feeds:
Feeding intolerance is a common cause for delaying introduction of progressive feeds in preterm infants. In LMICs, where resources or expertise to diagnose and confirm NEC maybe unavailable, feed intolerance defined as increased gastric residuals, emesis or abdominal distension(Khashana and Khashana A, 2016), is generally used as a predictor or initial manifestation of NEC. In the Cochrane review, pooled data from the eight trials that reported NEC, showed no evidence that delaying introduction of progressive feeds exceeding 24ml/k/day beyondfour days after birth compared to less than four days, reduces risk of NEC (RR 0.93, 95%CI:0.64,1.34).A recent RCT supports the same findings in ELBW infants(Salas et al., 2018). These findings show that progressive feeds could be given earlier, however, the ability to LMIC is unclear because the trials were undertaken in middle-or high-income countries where resources are available and stringent monitoring is practiced. The three trials that reported feed intolerance also showed no significant difference (RR0.84, 95%CI: 0.62,1.15), or on mortality (RR1.18,95%CI:0.75,1.88). It is worthy to note that feed intolerance was not well defined in the review hence caution is warranted when applying this finding. In clinical scenarios in LMICs, this is important because feed intolerance is putatively considered an early predictor of NEC(Li et al., 2014).The included trials had methodological weaknesses with unclear risk of biases in randomization and allocation concealment, and unmasked carers and clinical assessors. The overall quality of this review based on AMSTAR was high. As mentioned previously, the only shortfall was in the reporting of funding sources of the trials. The review provided adequate investigation of heterogeneity, appropriate methods for statistical analysis and adequate risk of bias assessment. Thus, we can be highly confident in the review results provided.
Recommendation: Although the findings suggest that early feeding soon after birth is safe for preterm feeding, the quality of evidence of the trials was low due torisk of biases. Caution is warranted applying these findings to LMICs and further high-quality trials is recommended.
Effect of minimal feeds on NEC:
The practice of giving small drops of milk into the infant’s mouth in the first few days of life may be protective against NEC. Benefits include stimulation of secretion of hormones and secretion of gastric enzymes, that promote intestinal maturation and adaptation in post-natal conditions(Li et al., 2008). In the Cochrane review, meta-analysis of nine trials involving 748 infants evaluated the effects of fasting versus small feeds on the incidence of NEC in VLBW infants. The findings demonstrated no evidence that minimal feeding of volumes up to 1ml/k/hour given from birth to one week, increases the risk of NEC(RR1.07, 95%CI:0.67,1.70) compared to fasting for the same period. This may also suggest that fasting does not prevent or reduce NEC, but the evidence is insufficient. There was also no evidence of effect on all-cause mortality (RR 0.66, 95%CI 0.41,1.07). The debate, albeit the mentioned findings, for LMICs has been associated with the contention whether to feed or not since these findings are unlikely to be generalised to LMICs. This underpowered study included30year old trials set in HICs on infants receiving high level care. In addition, the trials had methodological weaknesses and no report on the quality of evidence. The quality of the review however was highly rated and we can have overall confidence in the results of the review. Recommendation: Based on the evidence from this high-quality SR that fasting confers no advantage, the author recommends minimal feeding is safe for all preterms to start at day 1 provided that the infant is stable. High-powered RCTs are required to confirm the true effects of the impact on NEC and mortality in LMICs.
Effect of continuous versus intermittent bolus(IB) feeding on NEC
In LMICs the current practice is bolus tube feeding for babies less than 34 weeks gestation. Gavage feeding by oro- or nasogastric tube is necessary because these infants are unable to coordinate suckling, swallowing and breathing. Literature suggests that continuous feeding may have advantage over intermittent bolus feeding. In this review, I sought to find out the effects of continuous feeding compared to intermittent as a feeding regimen, its impact on the prevention of NEC, and if this method would be optimal for LMIC. In the included Cochrane review, it showed no difference in continuous feeding compared to bolus feeding on NEC risk (RR1.53, 95% CI:0.40,5.89)(refer Table 3).It concluded that there was inadequate evidence to determine which feeding strategy was optimal. In a recent RCT similar findings were also detected but interestingly, the trial showed lower levels of gastric residuals in the bolus group compared to the continuous group suggesting preference for the former if feeding tolerance was a concern(Rovekamp-Abels et al., 2015). In another RCT, the findings were similar showingno significant difference in weight gain and time to achieve full feeds between the preterm fed either by bolus or continuous methods(Star et al., 2012). The Cochrane review included small old trials with methodological flaws thus, it is difficult to draw conclusions of the best feeding method for LBW infants. Overall, the review was appraised to have moderate quality. While it reported ROB assessment for each primary study, it failed to mention the settings, and funding sources of both the included trials and the review. This is important as reviews with funding can have certain influences on the studies. Documenting the setting for the trials is helpful for applicability of the results to the setting in question. All other critical domains were fulfilled. Recommendation: Continuous feeding may not be a choice for LMICs as this type of feeding requires special syringes and pumps which in such settings may not be available. Moreover, whilst the quality of the SR was moderate suggesting that the results may be accurate, the available pooled data was not able to discern the clinical risks and benefits of either feeding methods and therefore further adequately powered, well-designed RCT sare required. An area for further research could also look at determining if either method could be tolerated with enteral feeds introduced from birth.
Effect of high volume feeds on NEC
WHO recommends maximum feed volumes of 180ml/k/d at two weeks for babies weighing 1000g, and one week for infants >1000g('WHO | Guidelines,' 2017). For a growing preterm, this volume maybe inadequate to meet all nutritional requirements, thus increasing the volume could make up for these nutritional deficits especially when multi-nutrient milk fortification may not be available or accessible. The review sought to determine ifgiving higher feeding volumes>200ml/k/day compared to the standard volume of 200ml/k/day influence NEC and if this feeding regimen would be optimal for LMICs. The included SR had only one RCT that addressed this question and NEC was a secondary outcome. The findings showed no statistically significant difference between the two regimens (RR1.03, 95% CI:0.07,15.78). While the data indicated that high volume feeding had significant effect on weight gain (primary outcome) without any adverse effects on NEC and feed intolerance (RR1.81,95% CI:0.89,3.67), the quality of evidence was low. The safety of high volume feeding was also demonstrated in an observational study (Klingenberg et al., 2017).This has clinical significance for LMICs as it could be an alternative strategy when nutrient fortifiers are not used. Moreover, the benefits of faster weight gain reduce complications associated with prolonged hospital stay and reduced nutrient intake. Overall, the review provided an accurate comprehensive summary of the trial results. A meta-analysis was still used for the single trial indicating it did not deviate from the protocol. The critical domains in the appraisal tool were satisfied however, like previous included SRs, trial funding source was unreported. Recommendation: Although the review showed robust methodology, the quality of evidence was low due to insufficient data thus caution is advised in interpreting these results. Larger RCTs are needed for this intervention especially for LMICs where supplementation with breast milk fortifiers is less feasible.

Human milk feeding
Effect of human milk versus formula milk on NEC
This review searched for SRs on the association of formula feeding on NEC in preterm infants compared to DM. FourSRs compare human milk to formula and their influence on NEC (Quigley, 2018, McGuire, 2003, Ben X, 2012, Miller, 2018).The meta-analysis suggests that DM may decrease the risk of NEC incidence by more than 60% and confirmed NEC by 75% (refer Table 4).The review by Ben X et al. (2012) investigating the same had the same trials including a trial in 2005. This review was also of low quality for the same reasons. Flaws in the methodology were; unreported funding sources, failure to declare any conflicts of interests, and a failure to state the existence of a protocol. The results demonstrated clear evidence of benefit for DM in reducing NEC.Quigley et al produced a high-quality review and confirms the same findings. All the required criteria were met giving it a high-quality status.Data from this review supports DM over formula for feeding. Feeding with formula milk almost doubles the risk of NEC (RR1.87,95% CI:1.23,2.85), but NEC in this review was a secondary outcome. The review by Miller et al confirms the similar results with evidence of moderate certainty of moderate risk reduction in NEC, in high dose milk(MOM+ DM) versus lower intake of HM (HM + formula)(RR0.59, 95% CI 0.29, 0.89)). Both Exclusive HM versus EPTF, and MOM versus DM(pasteurized) showed no difference. Whilst this SR demonstrated rigorous methodology providing a comprehensive summary of the available results,the findings from the trials were inconsistent. The trials were few to detect differences, and there was considerable heterogeneity. The question of whether fortification of HM has effects on NEC in preterm infants was examined in one SR. Brown et al included trials from all settings including LMICs, where NEC was a secondary outcome. This Cochrane review did not select studies in duplicate and did not indicate if trial funding sources were searched. Thus, the methodological quality was moderate suggesting it may have provided an accurate summary. Meta-analysis from the pooled data produced low quality evidence that fortification had no positive impact on NEC. In a RCT comparing human versus bovine-based HM fortifier on the incidence of NEC, results showed a significant difference with a reduction of 50% in NEC, and 90% in surgical NEC favouring HM-based fortifier(Sullivan et al., 2010). In a more recent trial(O'Connor et al., 2018), the findings contrast this and suggests that HM-based fortifiers did not improve feeding tolerance or reduce mortality and morbidity including NEC compared with bovine-based fortifier. The evidence on the optimal HM fortifier for feeding preterm is not discussed in this review however future research is warranted for LMICs. Overall, the clinical significance of the above findings supports DM over formula, and having more HM gives protection against NEC than having none. In LMIC settings where mother’s milk may be unavailable or insufficient due to factors such as poor maternal health, prolonged hospital stays or maternal-infant separation, donor milk is the next best choice. However, DM may not be feasible in such settings.DM banking processes include donor screening, pasteurization and storage and therefore could potentially be more expensive than formula milk. A SR that evaluated costs of DM compared to treating NEC showed DM cut costs by reducing incidence of NEC(Buckle and Taylor, 2017). It may therefore be cheaper than costs of prolonged hospital stay. Clinical decision makers may have to find alternatives to improve mother’s own milk supply, and even consider usage of fortifiers if required or available. Recommendation: DM is the next best option to MOM to reduce the risks of NEC so long as it is safe. Feasibility studies in LMICs on safe and cost- effective ways to improve human milk supplies are recommended. Larger RCTs with robust methodologies are required to confirm the true effects.
Effect of Lactoferrin
The literature offers a promising outlook for the role of oral Lactoferrin in NEC prevention. No adverse events have been reported in clinical trials done so far on preterm infants(Sherman et al., 2016). Several ongoing trials such as the LIFT trial(Ochoa et al., 2015), the ELFIN trial 2018 are underway assessing the association of lLactoferrin and NEC, but there are insufficient SRs. The included review by Pammi et al was assessed to be of low quality owing to inadequacy of investigation, and discussion of publication bias, and its impact on the results. This was a critical weakness in the methodology (refer Table 4).The meta-analysis showed a risk reduction of 60%, 95%CI(0.18,0.86),without any adverse events compared to the placebo group. This effect size is huge and of clinical importance albeit of low quality evidence. Lactoferrin appears to be safe and has the potential to reduce NEC in preterm infants thus, further research is needed to support these findings. The results can be generalised to LMICs as trials were also conducted in these set-ups, but more trials are needed to evaluate human Lactoferrin rather than bovine Lactoferrin. Recommendation: We await ongoing trials that not only can confirm safety and efficacy of Lactoferrin in VLBW or SGA babies but those that can compare human and bovine Lactoferrin, confirm dosing and duration, and possibly those assessing the possibility of emergence to resistance due to its antimicrobial properties. These are important for LMICs where factors such as cost effectiveness, availability, and safety are of primary concern.
Effect of probiotics:
Despite the extensive research on Probiotics, unanswered questions and controversies surrounding Probiotic use in preterm infants remain. Strain type, doses and duration of therapy, and the risks of sepsis are still to be determined. In this review, five recent SRs evaluated the impact of Probiotics on NEC (see Table 3). One of the limitations in this review was the exclusion of potential SRs that met the inclusion criteria but were excluded based on poor methodological quality. Across all included SRs, the effect estimates show consistent statistical significance in reduction of NEC. The reviews enrolled many trials with large numbers of participants. The mean effect estimate overall is around 50% reduction infrequency of NEC. Similarly, all-causes mortality in the five reviews show evidence of benefit in the reduction of deaths except for the review by Thomas 2017. The impact on ELBW infants contrasts these findings. Data from Alfaleh (2014), produced no evidence of effect on NEC in this cohort(RR of 0.76,95% CI: 0.37,1.58), and similarly, Thomas (2017),RR of 0.86(95% CI:0.64,1.16). These findings may not be clinically significant to LMICs as this cohort usually do not survive in these setups. Whilst only one review did not mention location, the rest had trials undertaken in both HICs and LMICs. Deshpande (2017) limited trials to LMICs, and meta-analysis had also confirmed statistical confidence that Probiotics added to feeds in preterm infants prevents NEC (RR 0.46, 95%CI 0.34,0.61)and reduce mortality (RR0.73, 95%CI 0.59,0.90). This large growing evidence from recent SRs demonstrates Probiotics can prevent NEC and consequently reduce mortality in preterm infants. This is promising however, even with this body of evidence, concerns exist regarding the precise strain type, and the possible infective risks as these are live bacteria given to infants with immature immune system. In the context of LMICs, several factors need to be considered. Quality control for instance, is important as these agents are not regulated under pharmaceutical companies. Moreover, contamination of these agents could be disastrous on a large scale if source is not identified early in Neonatal Units (NNU). Furthermore, consent by parents to allow treatment to be given would be another hurdle. Until such measures are in place, and concerns addressed, LMICs may not be ready to implement this intervention in the near future. The inconsistency in results could be explained by the difference in pathogens as the gut Microbiome is influenced by the mode of delivery, environment, breast feeding and exposure to antibiotics. Thus, until further research confirms these, Probiotics cannot be an optimum intervention in reducing NEC.
4.2 Strengths
The strengths of this review include the coverage of multiple interventions on NEC prevention in one review. While it was perceived at the outset to be a huge undertaking, the aim was mainly to synthesize a summary of the current evidence in one review to inform clinicians especially in LMICs to avoid searching through a plethora of RCTs. RCTs provide the optimum evidence when comparing interventions thus the review’s exclusive focus on SRs of RCTs or quasi-RCTs. Another perceived strength was the inclusion of mostly recent SRs including more Cochrane than non-Cochrane reviews. The reviews provided current evidence that may be more applicable today. The search was comprehensive in all relevant databases, grey literature and reference lists. I have not found a review on this topic of this design.
4.3 Limitations
There may have been some selection of review bias as there were potential SRs that met the inclusion criteria but were excluded due to the age of the participants. Two SRs assessed antenatal steroids on infants less than25 weeks. These were discarded due to the author’s opinion on viability and survival of this cohort in LMICs. In hindsight, RCTs for antenatal steroids could have been used to evaluate the effects on NEC, but this would have made this review more difficult to manage. Similarly, it is possible that some relevant SRs on Probiotics may not have been identified. These could provide some useful information on answers to the existing questions on safety for example. Another important limitation was not having a second reviewer for selection of reviews, data extraction, and to quality assess the included SRs. It was time consuming to keep returning to the articles to recheck information to ensure data was correctly recorded. A second reviewer would have solved this problem for validity and reliability of the review. Due to insufficient funds, this was not possible. Lastly, the realization that the project was enormous to be undertaken in a short time period was felt during the analysis process, but it was difficult to reduce after all the hard work put in. Not only was this exercise for my dissertation but for myself as a clinician to inform my practice in a low-income setting. Nonetheless, the work put in was thorough.
4.4 Implications for research.
Research gaps exist in antenatal steroids and its effects on NEC in all preterm infants. Although this is now standard current protocol for preterm labour in many units, true effects on NEC incidence needs to be determined. Moreover, further research on Lactoferrin is required and this intervention maybe more acceptable for LMIC given the dilemma with Probiotics. In summary, only two SRs were focused on LMICs. This reflects the need for more research in LMICs where most preterm infants are born and die from preventable but devastating conditions such as NEC.
5.Conclusion
NEC is likely a silent killer of preterm infants in LMICs. The burden of this disease is expected to increase with the rising number of preterm births and potential numbers surviving beyond the first few days of life. The findings from this review offer explicit conclusions and the author makes recommendation for practice in LMICs. Firstly,from the high-quality reviews, minimal feeds, rapid advancement, and early introduction of progressive feeds given as intermittent bolus feeding are safe feeding regimens for preterm feedingand are optimal interventions for LMICs. Secondly, high quality SRs confirm the superiority of human milk over formula in preventing NEC and suggest donor pasteurized milk as an alternative option in circumstances where mother’s milk is not available or cannot be given due to health. LMICs should invest in safe cost-effective strategies to improve human milk supplies, and acceptability of this intervention in these settings. Thirdly, human milk fortification is an option LMICs could use as rescue supplements for growth but its impact on NEC are research priorities. Lastly, while the roles of Lactoferrin and Probiotics in prevention of NEC are promising, there are research gaps and these pose challenges of generalizing the interventions to LMICs. In conclusion, the mentioned interventions are low-cost pragmatic interventions that may reduce NEC in preterm infants in LMICs. Implementing these interventions alone and the impact on NEC prevention in LMICs are future research opportunities. There is an urgent call for more research in LMICs if we are to improve the outcomes of preterm infants, and consequently the mortality rates in developing countries.
References
- Albenzio, M., Santillo, A., Stolfi, I., Manzoni, P., Iliceto, A., Rinaldi, M. and Magaldi, R. (2016) 'Lactoferrin Levels in Human Milk after Preterm and Term Delivery', American Journal of Perinatology, 33, pp. 1085-1089. doi: 10.1055/s-0036-1586105.
- Ballot, D. E., Chirwa, T. F. and Cooper, P. A. (2010) 'Determinants of survival in very low birth weight neonates in a public sector hospital in Johannesburg', BMC Pediatrics, 10, pp. 30-30. doi: 10.1186/1471-2431-10-30.
- Battersby, C., Santhalingam, T., Costeloe, K. and Modi, N. (2018) 'Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review', Arch Dis Child Fetal Neonatal Ed, 103(2), pp. F182-f189. doi: 10.1136/archdischild-2017-313880.
- Bergmann, H., Rodriguez, J. M., Salminen, S. and Szajewska, H. (2014) 'Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report', Br J Nutr, 112(7), pp. 1119-1128. doi: 10.1017/s0007114514001949.
- Berrington, J. E., Hearn, R. I., Bythell, M., Wright, C. and Embleton, N. D. (2012) 'Deaths in preterm infants: changing pathology over 2 decades', J Pediatr, 160(1), pp. 49-53.e41. doi: 10.1016/j.jpeds.2011.06.046.
- Braga, T. D., da Silva, G. A., de Lira, P. I. and de Carvalho Lima, M. (2011) 'Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial', Am J Clin Nutr, 93(1), pp. 81-86. doi: 10.3945/ajcn.2010.29799.
- Brown, J. V., Embleton, N. D., Harding, J. E. and McGuire, W. (2016) 'Multi-nutrient fortification of human milk for preterm infants', Cochrane Database Syst Rev, (5), p. Cd000343. doi: 10.1002/14651858.CD000343.pub3.
- Buckle, A. and Taylor, C. (2017) 'Cost and Cost-Effectiveness of Donor Human Milk to Prevent Necrotizing Enterocolitis: Systematic Review', Breastfeeding Medicine, 12(9), pp. 528-536. doi: 10.1089/bfm.2017.0057. Available at:
://WOS:000415088200007. - Caplan (2018) 'Necrotizing Enterocolitis in Preterm Infants is Related to Enteral Feeding, But the Mechanisms Remain Uncertain and Have Changed Over Time | SpringerLink', 2(4), pp. 241-247. doi: 10.1007/s40124-014-0062-8.
- Carter, B. M. and Holditch-Davis, D. (2008) 'RISK FACTORS FOR NEC IN PRETERM INFANTS: HOW RACE, GENDER AND HEALTH STATUS CONTRIBUTE', Adv Neonatal Care, 8(5), pp. 285-290. doi: 10.1097/01.anc.0000338019.56405.29.
- Chang, H. Y., Chen, J. H., Chang, J. H., Lin, H. C., Lin, C. Y. and Peng, C. C. (2017) 'Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis', PLoS One, 12(2), p. e0171579. doi: 10.1371/journal.pone.0171579.
- Choi, Y. Y. (2014) 'Necrotizing enterocolitis in newborns: update in pathophysiology and newly emerging therapeutic strategies', Korean J Pediatr, 57(12), pp. 505-513. doi: 10.3345/kjp.2014.57.12.505.
- Coutsoudis, I., Petrites, A. and Coutsoudis, A. (2011) 'Acceptability of donated breast milk in a resource limited South African setting', Int Breastfeed J, p. 3.
- Gephart, S. M., McGrath, J. M., Effken, J. A. and Halpern, M. D. (2012) 'Necrotizing enterocolitis risk: state of the science', Adv Neonatal Care, 12(2), pp. 77-87; quiz 88-79. doi: 10.1097/ANC.0b013e31824cee94.
- Hunter, C. J., Upperman, J. S., Ford, H. R. and Camerini, V. (2008) 'Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC)', Pediatr Res, 63(2), pp. 117-123. doi: 10.1203/PDR.0b013e31815ed64c.
- Kantorowska, A., Wei, J. C., Cohen, R. S., Lawrence, R. A., Gould, J. B. and Lee, H. C. (2016) 'Impact of Donor Milk Availability on Breast Milk Use and Necrotizing Enterocolitis Rates', 137(3). doi: 10.1542/peds.2015-3123.
- Lee, A. C., Katz, J., Blencowe, H., Cousens, S., Kozuki, N., Vogel, J. P., Adair, L., Baqui, A. H., Bhutta, Z. A., Caulfield, L. E., Christian, P., Clarke, S. E., Ezzati, M., Fawzi, W., Gonzalez, R., Huybregts, L., Kariuki, S., Kolsteren, P., Lusingu, J., Marchant, T., Merialdi, M., Mongkolchati, A., Mullany, L. C., Ndirangu, J., Newell, M. L., Nien, J. K., Osrin, D., Roberfroid, D., Rosen, H. E., Sania, A., Silveira, M. F., Tielsch, J., Vaidya, A., Willey, B. A., Lawn, J. E. and Black, R. E. (2013) 'National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010', Lancet Glob Health, 1(1), pp. e26-36. doi: 10.1016/s2214-109x(13)70006-8.
- Li, Y. F., Lin, H. C., Torrazza, R. M., Parker, L., Talaga, E. and Neu, J. (2014) 'Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance?', Pediatr Neonatol, 55(5), pp. 335-340. doi: 10.1016/j.pedneo.2014.02.008.
- Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., Clarke, M., Devereaux, P. J., Kleijnen, J. and Moher, D. (2009) 'The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration'. doi: 10.1136/bmj.b2700.
- Manzoni, P., Rinaldi, M., Cattani, S., Pugni, L., Romeo, M. G., Messner, H., Stolfi, I., Decembrino, L., Laforgia, N., Vagnarelli, F., Memo, L., Bordignon, L., Saia, O. S., Maule, M., Gallo, E., Mostert, M., Magnani, C., Quercia, M., Bollani, L., Pedicino, R., Renzullo, L., Betta, P., Mosca, F., Ferrari, F., Magaldi, R., Stronati, M. and Farina, D. (2009) 'Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial', Jama, 302(13), pp. 1421-1428. doi: 10.1001/jama.2009.1403.
- O'Connor, D. L., Kiss, A., Tomlinson, C., Bando, N., Bayliss, A., Campbell, D. M., Daneman, A., Francis, J., Kotsopoulos, K. and Shah, P. S. (2018) 'Nutrient enrichment of human milk with human and bovine milk–based fortifiers for infants born weighing< 1250 g: a randomized clinical trial', The American journal of clinical nutrition, 108(1), pp. 108-116. doi: 10.1093/ajcn/nqy067.
- Rozé, J.-C., Ancel, P.-Y., Lepage, P., Martin-Marchand, L., Nabhani, Z. A., Delannoy, J., Picaud, J.-C., Lapillonne, A., Aires, J., Durox, M., Darmaun, D., Neu, J. and Butel, M.-J. (2017) 'Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants', American Journal of Clinical Nutrition, 106(3), pp. 821-830. doi: 10.3945/ajcn.117.152967.
- Sankar, M. J., Natarajan, C. K., Das, R. R., Agarwal, R., Chandrasekaran, A. and Paul, V. K. (2016) 'When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries', J Perinatol: Vol. Suppl 1, pp. S1-S11.
- Sherman, M. P., Adamkin, D. H., Niklas, V., Radmacher, P., Sherman, J., Wertheimer, F. and Petrak, K. (2016) 'Randomized Controlled Trial of Talactoferrin Oral Solution in Preterm Infants', Journal of Pediatrics, 175, pp. 68-+. doi: 10.1016/j.jpeds.2016.04.084. Available at:
://WOS:000380835500017. - Tanner, S. M., Berryhill, T. F., Ellenburg, J. L., Jilling, T., Cleveland, D. S., Lorenz, R. G. and Martin, C. A. (2015) 'Pathogenesis of Necrotizing Enterocolitis: Modeling the Innate Immune Response', Am J Pathol: Vol. 1, pp. 4-16.
- Sullivan, S., Schanler, R. J., Kim, J. H., Patel, A. L., Trawoger, R., Kiechl-Kohlendorfer, U., Chan, G. M., Blanco, C. L., Abrams, S., Cotten, C. M., Laroia, N., Ehrenkranz, R. A., Dudell, G., Cristofalo, E. A., Meier, P., Lee, M. L., Rechtman, D. J. and Lucas, A. (2010) 'An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products', J Pediatr, 156(4), pp. 562-567.e561. doi: 10.1016/j.jpeds.2009.10.040.
- Thomas, J. P., Raine, T., Reddy, S. and Belteki, G. (2017) 'Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: a meta-analysis and systematic review', Acta Paediatrica, 106(11), pp. 1729-1741. doi: 10.1111/apa.13902. Available at:
://WOS:000412729900006.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



