Photochemistry and Light-Matter Interactions
MAIN BODY
Photochemistry can be described as; chemical reactions or physical changes that are as a result of matter interacting with light (particularly within the range of ultraviolet and the visible light spectra). [1] This principle uses quantum mechanics to explain the relationshop between light and matter. It is a very essential feature in chemical reactions that occur both in vivo (e.g. photosynthesis, in the formation of peptides, amino acids) and within the environment. And now through its resourcefulness it is being used in healthcare to improve on the quality of life. Photochemistry is used in biochemistry, biosurgery, molecular biology and oncology. [2] The principals of photochemistry can also be observed in two photon microscopy. Since photochemistry involves the interaction between light and matter, quantum mechanics can be used to explain this interaction. Through quantum theory we understand how electrons in matter behave as both wave and particle depending of the conditions in which it is being observed. [3] Quantum theory also shows that the energy in matter is quantised; that is only particular energies. Thus, quantised energy levels of matter have a separation that is of the same order as the energy of visible or ultraviolet light. [4] Therefore, the absorption of light within the mentioned spectra by matter will result in electrons being excited to higher energy levels, leading to the production of electronically excited species. Since light is also quantised, the absorption or emotion of light occurs by the transfer of energy as photons. [5] These photons display both particle like and wav like behaviours and each photon has a specific energy, E, given by Planck’s law: E=hʋ. Where h denotes Planck’s constant and ʋ is the frequency of the electromagnetic magnetic (EM) radiation that has been absorbed or emitted. [6] The term hʋ is used in photochemical reaction equations to represent a photon. Each photon oscillates with a wavelength λ, where λ=c/ʋ, c denoting the speed of light. Thus it can be seen that E=hʋ=hc/λ. The energy of a photon is proportional to its frequency and inversely proportional to its wavelength. [7] This means that light of a short wavelength corresponds to high energy.
There are two basic components required in photochemical reactions: the source of light (which enables the production of photons) and matter (e.g. the biological molecules), which are usually the target molecules. The target molecules are able to react with the high energy species produced in the process. In some cases, a third component usually arises; photo cross linker, photo initiator or a photosensitizer. [8] The third component’s function is to act as a mediator to the photochemical reactions. When it comes to choosing the source of light, there are two considerations as to the specificity of the wavelength. First, different depths into a target molecule are penetrated by different wavelengths; for instance, shorter wavelengths reach shallower layers, compared to longer wavelengths. This, however, is not a limiting factor when it comes to the application of photochemistry in some biology fields such as molecular biology and biochemistry; this is because most of these chemical reactions occur in dilute solutions. [9] Compared to these fields, the target molecules are usually denser in fields such as oncology, tissue engineering and biosurgery. According to Chan [10], a determination of the maximal effective optical penetration is necessary before the use of photochemical cross linkers. The maximal effective optical penetration refers to the maximum possible depth that can be achieved by respective photons in a light- interacting medium.

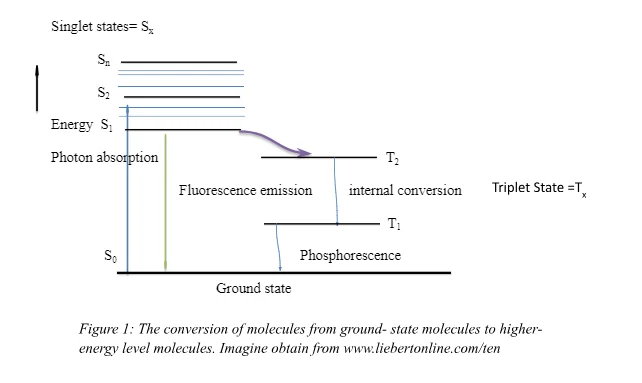
The second basic component is the target molecules. In order for a photochemical reaction to occur, target molecules have to be present so that they can absorb the light from the light source. These target molecules are usually application dependent because they are molecular moieties targeted by the photochemical reactions. [11] According to Levine [12], the molecule, after absorption, contains higher levels of energy compared to the ground-state molecule; it is said to be in an excited state (See figure 2). The target molecules have to be able to react with the photoproducts or the reactive species that arise as a result of photon absorption. Through this process, the target molecules undergo a variety of processes, both physical and chemical. Some of these processes would entail phosphorescence, fluorescence, generation of heat and formation of photoproducts. In addition to the components of the process, interfering molecules/ third party molecules may affect the effectiveness of these photochemical reactions. [13] A case in point is the presence of melanin as interference to the process in which light interacts with collagen in skin tissues. [14]
The third component in a photochemical reaction is the photosensitizer. Photosensitizers come in play in instances where the target molecules cannot be activated to produce the necessary photochemical reactions or fail to absorb certain wavelengths of light. Photosensitizers in such cases act to stain or bind the target molecules. Through this, these fluorophores are able to mediate the photochemical reactions with the preceding light absorption. There has been continuous development of photosensitizers throughout different generations and disciplines. There are specific optical properties associated with different photosensitizers; emission, absorption and fluorescence across the light spectrum. In order to assure maximum absorption, these respective optical properties have to be matched with respective wavelengths. [15]
Photocaging
Take a deeper dive into Pollution Dissertation Examples with our additional resources.
Biological processes (high processes that regulated naturally with very high spatiotemporal resolution in cells) tend to occur naturally in both single- celled organisms and multicellular organisms. [16] Activity in naturally occurring biological processes is indubitably controlled by a particular location and timing. This can be seen in complex biological processes occurring during an organism’s development (e,g by formation of peptides) . It is essential to control these processes with the same level of spatiotemporal natural resolution in order to understand them, together with their misregulation in human disease. Henceforth, light irradiation represents a unique chemical tool, as it can be controlled easily in location, amplitude and timing. For that reason, photochemistry plays a major role in the activation and deactivation of functions in biology. In order to achieve photochemical control over a biological molecule, the biologically active molecule can be rendered temporarily inactive via chemical modification with a photoremovable protecting group (PPG) in this context, a light removable protecting group. The compound protected by the light- removable group is called the ‘caging group’. [17] Barltop et al in 1962 [18] reported a photochemical deprotection reaction of glycine being released from N-benzyoxycarbonly gycine:

This then lead to advancement of other photoremovable protecting groups. Kaplan [19] used the word ‘cage’ to describe the molecules deactivating effect on the biological substrate that it is bonded to. To be removed by exposure to light. For the photoremovable protecting group to effective, it should possess several desirable properties. Sheena, Umezawa, [20] Lester and Nerbonne [21] initially provided a series of standards for evaluating the effectiveness of a PPG in set conditions. These properties include:
In cases where biological study is needed photoproducts, substrate or caged substrate need to soluble in an aqueous medium. However, in synthetic applications it is not necessary.
Photochemical release must be efficient (e.g. Φ˃0.10).
The primary photochemical process should release the cage chromophore from the photoremovable protecting group (i.e. it should occur straight after the cage chromophore is excited ).
In the photolysis environment, all the photoproducts need to be stable
The media, substrate or photoproduct should not absorb excitation wavelengths which should be longer than 300nm. I.e. In samples such as brain tissue or single living cells, to study the kinetics of rapid responses. Thus to diminish the intensity of the incident light which will reduce the risk of cell damage chromophores with a large photolysis quantum yield (Φ) and large molar absorptivity (ε) is used.
The addition of the PPG to a substrate must have a generally high-yielding synthetic procedure.
When a caged substrate is being synthesized, the separation of PPG and caged substrate derivatives must be quantitative. In deprotection process synthetic applications, this is also a necessary.
o-Nitrobenzyl
The most commonly used photoremovable protecting group (PPG) are nitrobenzyl, nitrophenethly compounds and their dimethoxy derivatives (nitroveratryl). The rate-determining step is not affected by the decay of their primary quinonoid intermediates. Potentially toxic and strongly absorbing byproducts such as o-nitrosobenzaldehde are formed during the photolysis of nitrobenzyls and its derivatives. [22] This has led to the development of other photoremovable protecting groups with few disadvantages.


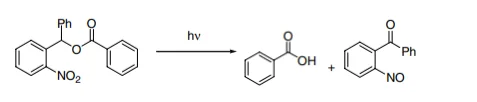
Figure 3, shows the release benzoic acid from the o-nitrobenzyl group. The low percent yield is a result of the conversion of the 2-nitrosobenzaldehyde and the initial photoproduct, this is the converted into azobenzene-2,2’-dicarboxylic acid. This then competes for the incident light. α-subsitituted nitrobenzyl esters increases the yield to between 75%-95% conversion. This is because the resulting photoprouduct is less reactive. (Figure 4)
The most common group of know glutamate receptors is N-methyl-D-aspartate (NMDA) uses the nitrobenzyl 2,2’-dinitrobenzhydryl as its photoremovable protecting group. DNB is used to esterify NMDA using a strong ultra violet absorber. Due to the poor solubility of DNB-NMDA in aqueous media, the addition of 20% Dimethyl sulfoxide (DMSO) to achieve complete dissolution. [23] The o-nitrobenzyl group has also been used in synthesis (in particular solid phase synthesis). The group attaches itself to the phosphate group of the nucleotide that is bound to a carboxylic acid through an alkyl chain.
Bezoin
The photochemical reactions of particular benzoin derivatives to produce 2-phenylbenzofuran was studied by Sheehan and Wilson. [24] α-Chloroacetophenones (an example of a group that is attached to the α carbonyl) are lost during these reactions. They went further to suggest one particular benzoin; 3’,5’-dimethoxybenzoinchromophore could be used as a photoremovable protecting group for carboxylic acids. In reactions where there were ungarnished benzoin cage, phosphates were quantitatively released. Subsequently, the parent chromophore’s range of application and nature are extended. [25] Benzoin and substituted benzoin esters are used as traceless linkers for solid phase synthesis of oligopeptides and the introduction of Fluorenymethyloxycarbonyl (Fmoc) - protected amino acids
Phenacyl
For the release of benzoic acid, several amino acid derivatives and peptides, Sheehan and Umezawa [26] used a version of the benzoin chromophore. The proposed photolysis mechanism involved the homolysis of the carbon-oxygen bond to yield p-methoxyacetophenone (photoprouduct). (Equation 1.) The hydrogen donor in this reaction is ethanol and in the presence of 1M benzophenone or naphthalene the reaction is quenched indicating a triplet reaction pathway.
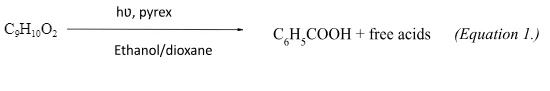
p-hydroxyphenacyl rapid release of substrates, enables fast biological processes to be studied. For example Givens et al. [27] applied the p-hydroxyphenacyl to the investigation of the bradykinin BK2 receptor.
Arylcarbonlymethyl
Since aromatic ketones have well known photophysical and photochemical properties; they are thermally stable and synthetically readily accessible compounds. Carbonly compounds tend to have low energy transition, which are weak,* band. [28] The internal conversion to the singlet state (S1) is fast and the absorption bands are strong for the higher-energy and *. Solvent polarity and substitution on the phenyl ring affects the electronic transitions of aromatic ketones. The and * states are often stabilized by both electron donating groups and polar solvents. The hypsochromic shift of the n, * absorption is band is brought about by the presence of the hydrogen bonding of the protic solvents to the carbonyl oxygen stabilizes the oxygen non-boding orbital. [29] The centre of the most of the photochemical reactivity is usually the carbonyl group of the aromatic ketones, with reactions leading to the release of the leaving group. [30] Ketones with n, * low triplets tend to pose a half vacant orbital that is localized on the carbonyl oxygen are much more reactive than those with spins delocalized on the aromatic ring.
Coumaryl and Arylmethyl
The rapid photofragmentation reactions of benzyl acetates are very rapid. According to Zimmerman [31] meta activation of the excited singlet state of benzyl acetates takes place through the approach of the excited and ground state energy surfaces, channelling the excited state toward heterolysis of the benzyl ester bond. Homolytic fission and radical derived products are as a result of the electron donors found at the para position. Due to favourable redox properties of the radical pair, the electron transfer of arymethyl derivatives substituted with a meta electron-donating group, occurs more rapidly than competing radical processes. In cases where the initial radical pair have rapid, more favourable divergent pathways available, the steps leading to the ion pair will play a significant role in the outcome of the photorelease. [32]
Two Photon Systems
Continuous development applications of photochemistry led to the development of two- photon absorption. This eventually increased the application of photochemistry in biological fields such as microscopy, optical power limiting, photodynamic therapy, and microfabrication, localized release of bio- active species and three dimensional storage of data. The theoretical aspect of the absorption of two photons by the same molecule was first analyzed by Göppert- Mayer in the 1930s. [33] As sub- picosecond pulsed lasers became more easily accessible, two- photon absorption became easier to investigate. The clear difference between the conventional one photon absorption and the two photon absorption is that the latter involves interaction of two photons, therefrom increasing the light intensity square. This intensity dependence has led to most applications for two photon absorption. Two –photon excitation microscopy is fluorescence imaging technique that enables the visualisation of living tissues at depth. [34] It provides significant advantages when employing the use of three-dimensional imaging and it is used as an alternative for confocal or conventional (one- photon) fluorescence and deconvolution microscopy. It is known widely by other names such as non-linear multiphoton, two-photon microscopy or two-photon laser scanning. The confocal microscopy allows for three-dimensional sectioning into thicker tissues. A pinhole is used to eliminate the out of focus background fluorescence. [35] Nonetheless the excitation of molecules still generates fluorescence. Therefore, leading to the production of photobleaching and phototoxicity throughout the specimen. [36] A large amount of excitation can cause a critical amount of photobleaching and phototoxcity problems which can be detrimental to live specimens. The best solution for specimens with relatively low overall signal levels or out of focus backgrounds, is to use deconvolution techniques. The excitation intensity is kept quite low as this method of microscopy uses a wide field microscopes for image acquisition. [37] As a result deconvolution microscopy is usually used for imaging monolayers of living cells. Only true constrained iterative deconvolution techniques produce quantitative data and that the deconvolution techniques use non-linear data filters that do not generate quantitative data. Due to the use of wide field microscopes for image acquisition, there is limited penetration into thick specimens. This is because there is an increase of out of focus background and light scattering. Also the large amounts of calculation requirement prevents immediate feedback during an experiment. [38] The two-photon absorption is a concept where by two photons of identical or difference frequency cause a molecule to be excited from one energy level (ground state) to a higher energy level in a singlet state. [39] This transition can simple be described as an electron moving from atomic orbital that is less stable. (Figure 5) The sum of the energies of the two photons absorbed is equivalent to the energy difference between the two states.
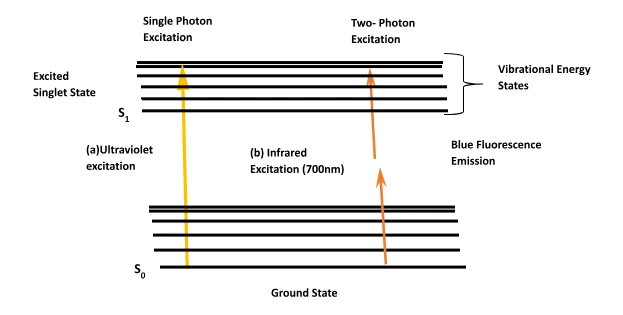
Since wavelength is proportional to energy (see equation 1); the shorter the wavelength the more energy it has. This is an inversely proportional relationship, thus a photon that has wavelength of 400nm has twice the amount of energy as a photon that has a wavelength of 800nm. The emitted fluorescence varies with the square of the excitation intensity, because the two photo excitation depends on simultaneous absorption. The photon density needs to more than one million times that required to generate the same amount of one photon absorption. This required so as to produce a significant number of two photon absorption, where both photons interact with the flurophore at the same.[40] Consequently, the amount of laser power needed to generate two photon excited fluorescence is high. However, the power needed can be attained by focusing pulsed lasers (mode-locked), where the power produced during the peak of the pulse is enough to generate the two photon excited states. While the average laser power remains low. [41] Thus, the same singlet state seen when carrying out conventional fluorescence experiment, is the same as the resulting two photon excited state from which emission occurs. There are certain fundamental principles of the two photon absorption. The equation that attenuates the beam of light resulting from two- photon absorption uses assumptions equivalent to those of the Beer Lambert law for the conventional one- photon absorption (Shown below); where I is the light intensity, z is the distance and N is the molecules number per unit volume, a2 represents a molecular coefficient for two- photon absorption. [42]
dI/dz = -N a2 I2= -N d F I
The theoretical analysis of the application of two- photon absorption, developed through experiments, concludes that there are certain requirements necessary for the maximization of the two- photon cross- section of a chromophore. Pawlicki et al [43] state that as a fundamental research in biology and life sciences, two photon initiated release of bioactive molecules is an extremely powerful tool. Application in the biological field of photo activation and drug deliver is aligned with the neurophysiologists’ interests in controlling neuronal circuits through realization of localized, rapid release of proteins, ions and neurotransmitters. Chromophores used in this context have extremely small two- photon absorption photons. The values are determined through measurement of the amount of femtosecond two- photon excited products resulting from the photochemical reaction. The conventional one photon absorption is used as a basis for the majority of dyes used in the two photon uncaging. 4-methoxy- 7- nitroindolino glutamate derivative, for instance, has resulted into various developed understandings of signalling of the neurons. Other two- photon dyes have also been explored, such as stilbene and photo labile masked glutamates, derived from coumarin. At single synapses, it is also vital for two- photon absorption- initiated release of calcium. This enables the analysis of molecular processes. In regards to drug delivery, a strategy that is commonly used for drug delivery is encapsulation in liposomes and micelles. [44] It is possible to selectively release high local concentrations of an active compound in specific diseased tissues, if this strategy can constructively be controlled by two photon absorption. Upon excitation, the diazo compound involved undergoes rearrangement in structure, which in turn increases its hydrophilicity, destroying the micelle. Photochemistry application in biology is also commonly seen in treatment modality to kill cancer cells. PDT uses photosensitizer and light to form photochemical reactions for that function. This, among other reactions, constitute some of the photochemistry applications in biology.

There are numerous advantages that arise by using the two photon in laser scanning microscopy. The first one is from the physical principal that the absorption is dependent on the square of the excitation intensity. The generation of the two photon excitation is achieved by focusing a single pulsed laser through the microscope optics. As a result the photons spatial density increase (simply put as the photons become more crowded), thus the probability of photons simultaneously interacting with the single fluorophore is increased. [45] However, the location along the optical path where the photons a high spatial density to increase the occurrence of two photon excitation is the focal point of the laser. Above this particular point photons will not be crowded enough for them to pass with the absorption cross section at the same time. The generation of the two photon excitation can also be achieved by utilizing the pulses available from the mode locked laser. Thus, combining this two factors will allow for the effective generation of the photon intensities needed for the two-photon excitation. The most significant advantage of the two photon microscopy of the confocal microscopy is the narrow localization of the two photon excitation. In the latter it is found that even though the fluorescence is excited throughout the specimen, only the signal that originated in the focal plane will pass through the confocal pinhole therefore allowing background data to be connected. [46] Another important advantage is that the three dimensional resolution of the two-photon excitation is similar to the one achieved through confocal microscopy. Also, since most of the excitation light penetrates through the specimen to the plane of focus there will be no absorption in the out of focus specimen areas thus increasing the specimen penetration. [47] The two photon microscopy also mitigates the damages caused by photobleaching and phtotoxicity (the two, most significant limitations to fluorescence microscopy of living cells). These two limitations often lead to the reduced viability of the biological specimens being investigated. The achieving of three dimensional resolution does not require a pinhole while using of two-photon excitation microscopy, this allows for greater flexibility in the detection geometry. [48]
The geometries for two photon microscopy are characterised as either descanned or non-descanned. In the former geometry the emitted light will return along the same path as the excited light, striking the scanned mirror before passing the confocal pinhole to the detector. [49] While the latter geometry is more suited for fast data acquisition. This is because there is the presence of a conjugate plane detector arrangement which allows for the locating of the dichroic mirror immediately after the objective lens. This will then reflect the emitted light through a transfer lens to a detector which is located in a plane conjugate to the rear aperture. [50] The need to collect the emitted light through an external detector directly from the specimen, passing through the objective lens is eliminated. The emitted light can also be diverted by using a dichroic reflector to a charged couple device (CCD) at the intermediate image plane. As mentioned early the reason why it is advantageous to two photon microscopy is because of its ability to obtain superior sectioning at greater depths during the analysis of thick specimen. This increased depth penetration is achieved using three physical mechanism. [51] Thus, when used to together increase the resolution when studying thick specimens. These include:
Allowing more of the excited light photons to reach the desired specimen by removing the out of focus absorption.
The use of red and infrared light (longer wavelength) in two photon excitation as opposed to blue and ultraviolet light (shorter wavelengths) which undergoes more scattering.
In confocal microscopy the effects of light scattering is more detrimental.
Photochemistry is used in day to day life, however the uses tend to vary depending on the application. It is a principle that outlines the relationship between light and biological molecules. The work has ensured that the components and mechanism of photochemistry have been revisited. Reviews on biomedical applications of photochemistry in various disciplines including and not limited to oncology, molecular biology and biosurgey. Emphasis was put on photocaging; with the different types of photoremovable protecting groups being looked at. In terms of their chemical and physical properties. The two photon microscopy was reviewed, the main principles were outlined. Examples of the uses as well as advantages over other systems were studied.
References
Chan, B. P. (2010). Biomedical Applications of Photochemistry. Tissue Engineering: Part B, 16(5), 510-518.
Wardle, B. (2009). Principles and Applications of Photochemistry. Manchester: John Wiely & Sons.
Furuta, T., Wang, S., Dantzker, J., Dore, T., Bybee, W., Callaways, E., . . . Tsien, R. (1999). Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sectionsfor two photon photolysis. Proc. Natl. Acad. Sci. USA, 96, 1193-1200.
Lee, H., Larson, D., & Lawrence, D. (2009). Illumination the chemistryof life: design, synthesis and applications of caged and related photo responsive compounds. ACS Chem Biol.
T. J. Gould, V. V. (2009). Imaging biological Structures with fluorescence photoactivation localization microscopy. Nat. Protocols.
Dawson, J. B., Barker, D. J., Grassa, D. J., Cotterill, J. A., Fisher, G. W., & Feather, J. W. (1980). A theoretical and experimental study of light absorption and scattering by invivo skin. Phys Med Biol.
Juzeniene, A., Peng, Q., & J. Moan. (2007). Biophysicalaspects of photodynamic therapy . Photochem Photobiol Sci.
Plaetzer, K., Krammer, B., Berlanda, J., Berr, F., & Kieslich, T. (2009). Photophysicsand photochemistry of photodynamictherapy: fundamental aspects . Lasers Med Sci.
Klan, P., Solomek, T., Bochet, C. G., Blanc, A., R, G., Rubina, M., . . . Wirz, J. (2012). Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanism and Efficacy . Chem Rev, 119-191.
Sheehan, J., & Umezawa, K. (1973). Phenacyl photosensitive blocking groups. J. Org. Chem.
Lester, H. A., & Nerbonne, J. M. (1982). Physiological and pharmacological manipulations with light flashes. Ann. Rev. Biophys. Bioeng., 151-175.
Sheehan, J., & Umezawa, K. Op cit.
Givens, R. S., Weber, J. F., II, P. G., Orosz, G., Donahue, S., & Thayer, S. A. (2000). New phototriggers. p-Hydroxyphenacyl as a Cterminal photoremovable protecting groupfor oligopeptides . J. Am Chem, Soc, 2687-2697.
Zimmerman, E. H., & Sandel, R. V. (1963). Mechanistic organic photochemistry II.Solvolytic photochemical reactions. J. Am. Chem. Soc., 915-922.
Pawlicki, M., Collins, A. H., Denning, R. G., & Anderson, H. L. (2009). Two- photon Absorption and the Design of Two-photon Dyes. Angew Chem Int.
Masters, B. R., & So, P. T. (2004). Antecedents of Two-Photon Excitation Laser Scanning Microscopy . Miccroscopy Research and Techniques .
Piston, D. W. (2006, July 13). The coming of age of two-photon excitation imaging for intravital microscopy . Advanced Drug Deliivery Reviews, pp. 770-772.
Deiters, A. (2010). Principels and Applications of the Photochemical Control Cellular Processes. Chembiochem, 11(1), 47-53.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



