Supply Of Oral Cancer Drugs In Uk's Community
Introduction
In 2010, the UK’s National Health Service published a white paper that advocated for the extension of patient choices and elimination of bureaucracy within care pathways (Crowe, et al., 2010). Consequently, chemotherapy service providers extended their services from primary and secondary care to community-level care, building upon previous NHS initiatives to deliver patient-centered care at community level. More importantly, the white paper formed the foundation for the shift from Primary Care Trust (PCT) to a GP-led commissioning in 2012 (Fife, 2011). Consequently, there was a general expectation that the NHS would commission all the anti-cancer drug supply services at community levels as part of the strategy to enhance NHS primary care services (Crowe, et al., 2010).

Against this backdrop, there has been a strong motivation among NHS commissioners to devolve services and move them closer to patients based on statistical predictions of an increased number of people requiring those services in future (Arlow, 2011). For instance, cancer chemotherapy was one of the services that faced capacity pressure due to the increase in number of patients undergoing chemotherapy treatments. As a result, alternative models of chemotherapy emerged, one of them being the cyclical dispensing at the Christie NHS Foundation Trust.
This model was adopted since there are various community healthcare professionals and pharmacists who had gone beyond the boundaries of their professional job descriptions to care for cancer patients. Furthermore, it was based on the realization that there were numerous community pharmacists who would be involved in dispensing, administering and monitoring cancer patients receiving anti-cancer care within the community (Erning, et al., 2016).
However, there have been increasing concerns over the safety of dispensing and supply of oral cancer drugs within the UK’s community especially after a safety alert issued in 2008 by the National Patient Safety Agency (NPSA). Particularly, this report highlighted the risks associated with incorrect oral anti-cancer drug dosages prescribed and dispensed (Verger, et al., 2017).
The risks associated with drug dispensing have been highlighted across pharmacy literature, leading to an increased need and awareness on the role of medicine dispensing in maintaining, protecting and restoring the lives of people depending on those drugs. The reason for concern are the onset of adverse health effects that have serious consequences on the health and well-being of patients as well as serious reputational damage on health institutions and health professionals (Kulesskiy, et al., 2016).
The art and science of maintaining drug safety has evolved over many years. Consequently, there have been radical changes in what can be considered a benefit or risk of drug safety as well as the safety requirements following various therapeutic development and disastrous outcomes associated with those development (Suda, et al., 2017). For instance, the United States of America (USA) experienced a major drug safety issue in 1937 with sulfanilamide elixir containing diethylglycol, triggering the Food and Drug Administration (FDA) to request toxicity essays as one of the requirements for sale of new drugs authority (Erning, et al., 2016). As a result, health authorities around the world have embarked on developing oversight measures to reduce drug-induced adverse effects as well as other drug safety surveillance measures to minimize effects on the general population. Consequently, drug dispensing models such as the cyclical dispensing at the Christie NHS Foundation Trust have come under the radar of regulators who assess their safety and cost effectiveness before authorizing their application.
Anticancer medication is given in cycles, as opposed to a single treatment. The cyclic medication is characterized by several breaks in between that combine to form a cycle of on and off oral drug taking (Kulesskiy, et al., 2016). Usually, a course of treatment could consist of up to 8 cycles on average. However, sometimes, treatment is extended over longer time this way to ensure efficiency and to allow the body to rest and recover. Although some of the cancer cells could also be resistant and thus chemotherapy might not be effective against those cells, as it only acts on cells that are in process of division. Having treatment in cycles makes sure that the cells that were in a resting mode during one of the cycles are killed off in the following cycles.
Many oral anticancer drugs treating different conditions are being supplied to the patients under the care of The Christie Hospital. The supply is done via a dispensing scheme, which has been recently implemented. Under the scheme those oral medicines are being supplied one cycle at a time and irrespective of patient review dates. It is though that this type of dispensing may bring positive outcomes, however no previous research on this has been done to show this.
The location of any drug dispensary has a significant influence on the safety and cost of drug dispensing which must be taken with high regard. However, while the Christie NHS Foundation Trust operations are heavily regulated and standardized, there is little evidence of the suitability of where they have located their dispensaries. Furthermore, it is unclear whether their medicine delivery services enable easy identification of persons delivering the medicines. Apart from location., other factors such as the dispensing environment and dispensing personnel are important and must be considered when exploring the safety and cost effectiveness of the cyclical dispensing at the Christie NHS Foundation Trust. Therefore, the main aim of this study is to evaluate the dispensing scheme at the Christie Hospital Foundation by looking the safety and const effectiveness measures set up by the trust to ensure safety and well-being of target patients.
Research Aim
To investigate the safety and cost effectiveness of cyclical dispensing at the Christie NHS Foundation Trust
Research objectives
To investigate the safety of cyclical dispensing at the Christie NHS Foundation Trust
To identify the cost effectiveness of cyclical dispensing at the Christie NHS Foundation Trust
To explore the factors influencing safety and const effectiveness of cyclical dispensing at the Christie NHS Foundation Trust
Justification of the study
Identifying the safety and cost effectiveness of cyclical dispensing at the Christie NHS Foundation Trust is an important policy and regulatory item that seek to ensure both health and economic well-being of cancer patients. Medicine dispensing is a health practice that involves various procedures, protocols, technology and management practices that form part of the system of operation at the Christie NHS Foundation Trust. These elements could be improved by identifying the level of safety and cost effectiveness of the entire system. Furthermore, cancer patients spend a lot of money on cancer medication and chemotherapy. Therefore, in the spirit of quality healthcare, the findings of this study will help identify the loopholes in such an important system that and develop interventions that may not only save on costs but also eliminate health risks that may emanate from unsafe dispensing service.
Research by Belfrage, et al. (2014) reveal that medical dispensing is one of the areas within the cancer care pathway that may experience rampant medication errors. While the prevalence of medication errors has been on a downward trend in the past few years (Kulesskiy, et al., 2016), the findings of this this study will help in brining further improvements within the UK’s pharmacy and drug distribution system. This is especially important considering that community pharmacies in the UK distribute large volumes of cancer medication (Belfrage, et al., 2014) that even a slight error can affect many people.
Kulesskiy, et al. (2016) insists that to guarantee safety among cancer patients on oral cancer therapies, there should be an intensified quality assurance and checking of drug prescriptions to reduce the incidences of medication errors. This study would support this initiative by producing empirical evidence on the extent to which cancer medication dispensing is safe and cost effective as well as giving recommendations on how its effectiveness can be improved. Furthermore, the findings of this study will help in the development of strategies for effective communication among the staff responsible for cyclical dispensing at the Christie NHS Foundation Trust, thereby helping to reduce administrative errors that could endanger patients’ lives.
Structure of the dissertation
After the introduction and setting the background of the study, the author will move into the literature review section, which identifies the existing literature on medical dispensing and its related safety and cost issues. Also, in the literature review section, the author will identify existing research gaps in this area of research and how the study fills these gaps. After the literature review, chapter three will be the research methodology section. Here, the author will identify the techniques, strategies and procedures through which the study objectives were achieved. In doing so, the author will state and justify various elements of research methodology such as research approach, research philosophy, data collection and data analysis among other elements. The next chapter will be the results. Here, the author will present and interpret results of the study. Finally, chapter five will entail a discussion of the results as well as a conclusion of the study.
Literature Review
Existing pharmaceutical research agree that effective medication dispensing entails giving medicine to the right person based on the correct prescriptions (Kaiser and Schmid). The dispensing pharmacist must correctly interpret the prescriber’s intentions and wishes so that they can accurately prepare and label the medicine for a safer use by the patient. Meanwhile, because medication dispensing may take place in many places including community shops, public or private clinics, hospitals and health centers, safety and cost effectiveness of medication dispensing has been explored in many research directions, with little success on what constitutes a safe and economically viable dispensing (Al-Rukban and Rizvi). In this section of the study, existing literature material on medication dispensing will be explored, what is known and what is yet to be established about the safety and cost effectiveness of medication dispensing. In the process, there will be a comparison of the different elements of cyclical dispensing at the Christie NHS Foundation Trust. Ultimately, the study will identify existing research gaps within the contexts of these elements and speculate whether the current research will close those gaps.
The principle of rational use of medicine (Al-Rukban and Rizvi) consider dispensing as one of its most important components. Programs such as the cyclical dispensing at the Christie NHS Foundation Trust hold with high regard the rational use of medicine with specific focus on dispensing and patient’s consumption of the medicine.
While dispensing is often perceived as a simple task that entails little risk of errors, all the resources involved in it can go into waste if the medicine does not end up in the hands of the person for which the drugs are prescribed, or if the person receives incorrect drugs or incorrect dosage advice. Consequently, researchers have been keen to evaluate factors that influence medication dispensing, which therefore ensures correct medication dispensing.
Dispensing Environment
(Lowery) asserts that pharmacists must ensure that they operate in a clean environment. This is particularly important for cancer medication because most of them are for internal use and therefore they must be uncontaminated and hygienic. Similar remarks are made by (Lahtela, Jylhä and Saranto) who insist that the accuracy and efficiency of dispensing depends on how the environment is organized. In this regard, (Lafata, Schultz and Simpkins) and (Brady, Wunsch and DiMaggio) consider the dispensing environment to be composed of the physical surrounding, the staff, packaging materials and equipment, work surfaces, storage and shelving. Moreover, all the staffs involved in dispensing should wear clean clothes/uniforms and maintain good personal hygiene. However, there is an unclear evidence on whether the cyclical dispensing at the Christie NHS Foundation Trust observes these environmental hygiene factors in their dispensing process. While the NHS Foundation Trust indicate that dispensing can either be done on shop or through a delivery services (Sjöberg, Edward and Fastbom), less has been established whether the delivery staff or the staff at their shops observe these hygiene protocols. Thus, through the current study, it would be interesting to address this grey area of knowledge by investigating the hygiene levels maintained by staff responsible for the cyclical dispensing at the Christie NHS Foundation Trust. Furthermore, there is a lack of evidence on the availability of any routine cleaning of dispensing surfaces and storage shelves implemented by Christie NHS Foundation Trust.
Another important element of dispensing environment that many studies have given attention to is the location of dispensaries. While the dispensaries must be located in easily accessible points, they should also be located in protected areas and not in open places such as close to reads where they are exposed to dust, pollution or dirt (Lee and Malone). Similarly, dispensaries should be in restricted areas where only the authorized persons can access. These observations bring to question the locations Christie NHS Foundation Trust’s dispensaries. While the Christie NHS Foundation Trust operations are heavily regulated and standardized, there is little evidence of the suitability of where they have located their dispensaries. Furthermore, it is unclear whether their medicine delivery services enable easy identification of persons delivering the medicines.
Existing research has also taken a keen interest in the dispensing equipment (e.g. those used in counting tablets and measuring liquids) as one of the most important elements of medication dispensing that would of interest to this study. For instance, uncoated tablets leave layers of powder on the dispensing surfaces that can easily be transferred to other tablets if the surfaces are not well-cleaned (Tora, Bo and Bodil). This may lead to cross-contamination and can be dangerous to the patient if the contaminating agent is one that the patient is sensitive to. Therefore, cleaning the dispensing environment and surfaces is an important process of medicine dispensing process that keeps patient safe.
An organized dispensing environment also another important part of dispensing safety. Organizing the dispensing environment regardless of the existing dispensing system is an important safety measure that cannot be ignored. In this regard, some pharmacists organize their stock shelves in dosage forms such as tablets, capsules, mixture sand syrups to ensure safe selection of medicine especially when patients come in person to buy the medicine (Wallerstedt, Fastbom and Johnell).
(Chou, Yip and Lee) observe that in most cases, people assume that dispensing only entails giving medicine to patients based on written prescriptions and therefore anyone can do the job without any professional qualification or supervision, an assumption that is dangerous and irrational. In this regard, supplying medicine and supplying goods differ in the sense that in the former case, the recipient may not have the expertise to judge the correct quality or know the correct use of the product they receive. Therefore, the responsibility of ensuring quality and correct use solely lies on the person dispensing them (Alam, Mishra and Prabhu). This justifies why in the UK and other jurisdiction; the law requires that dispensing and distribution of medicine is conducted by professionally qualified pharmacists. However, where there is a shortage of qualified pharmacists, dispensing and delivery of medicine may be left to individuals with no training in medicine or in the safe use of medicine (Sweidan, Reeve and Brien). Moreover, medicine dispensing, and distribution requires pouring, counting, and writing skills in addition to the right attitudes, knowledge and mentality to complete the dispensing process. Similar observations are made by (Nasser, Nassif and Mahfouz) who identify five basic skills that every individual tasked with dispensing or distributing medicine must have. First, they must have enough knowledge about the medicine being dispensed, including the correct dosage, methods of use, storage guidelines, and interactions with food and beverages. Secondly, the person must have arithmetic and calculation skills as well as skills for quality preparation of the medicine (Whitehill, Wright and Robinson). They must also have the attributes of honesty, cleanliness and accuracy. Lastly, persons tasked with dispensing medicine must have the right attitude and skills for effective communication (Mittal, Mittal and Singh). In the context of the current study, it is still difficult to establish whether the Christie NHS Foundation Trust’s staff tasked with implementing the cyclical medical dispensing system have all these skills and attributes. It would be interesting to find out the skill gap among the staffs and the necessary interventions put in place by the Trust to equip them with the skills.
Dispensing staff must be well-trained to oversee a correct dispensation of medicine as prescribed in the facility. Regardless of working in the public or private sector, professional training on basic pharmaceutical handling skills is important (Borel and Rascati). Some NHS Trusts have a limited range of prescribed medicine and a small number of handled patients and thus they may only require basic but highly structured training on dispensing that is based on the practitioners’ background health training (Chapuis, Roustit and Bal). However, dispensing assistants with basic level training can also be employed in high ranking hospitals but should be supervised by trained pharmacist (Kamuhabwa and Silumbe).
Dispensers who work in community pharmacies should also have a similar level of training that highlights the basic practices of dispensing and medicine handling (Lindh, Andersson and Mannheimer). It may also be useful to train them on basic medication adherence counselling in readiness for handling with poor medication adherence behaviors. Therefore, while the reviewed literature highlights the importance of dispenser training and education, there is little knowledge on whether the Christie NHS Foundation Trust has a training framework in place for its staff to equip them with the necessary skills they need in implementing the cyclical medicine dispensing. Furthermore, it would be interesting to identify whether the staffs have the necessary skills and knowledge to handle community level challenge of medicine non-adherence.
Theoretical Approaches to Medication dispensing
Many theories have been developed to best understand the different modalities of medication dispensing that would ensure safe and cost reduction while enhancing patient adherence. For instance, behavioral theories that have been to understand human behaviors can also be used in understanding pharmacist’s actions of cyclical medicine dispensation.
Theory of planned behavior (TPB)
TPB has been used by some scholars to understand how pharmacists interact with patients when dispensing drugs. Particularly, believers in the TPB argue that developing a behavioral intention and actual performance of the behavior depends on three major factors namely the individual’s behavioral attitude, perceived social pressures from other people’s behaviors and expectations, and one’s perceived ability to control the behavior (Chen and Wu). If the individual has more favorable subjective norms and attitudes towards the behavior as well as perceive control over the behavior, they are more likely to practice that behavior when an opportunity arises (Kaye, Sands and Donahue). Thus, regardless of any ethical or legal implication, scientist have attempted to use the TPB to analyze and understand what drives human behavior. Similarly, TPB has been used to understand health practitioners’ behaviors towards patients, including how they communicate about drug abuse and misuse especially with regards to opioids and pain relievers (Romero and Malone). Similar observations are made in (Fontan, Maneglier and Nguyen) who use TPB to understand pharmacists’ intention to educate patients on drug disposal and prescription drug monitoring programs.
Because there is a paucity of studies that have used TPB to understand the Christie NHS Foundation Trust’s cyclical dispensing, the current study will apply the TPB theoretical framework in explaining cyclical dispensing as a behavior-specific intervention plan and evaluate the extent to which pharmacists’ attitudes, perceived behavioral control abilities and subjective norm beliefs affect patient safety as they use the cyclical dispensing model.
Integrated dispensing Model
Some scholars (Mekonen, Samuel and Ambelu) and (Mertens, Kwint and Belitser) argue that to achieve rational drug use, drug dispensing should be conducted in a model that is integrated into the care process with key considerations on accessibility of the drugs, accountability, connection and accountability, as well as clinical pharmaceutical as illustrated in the figure below:
Ideally, this model works based on service system frameworks that explore the pharmacists’ roles and the importance of effective drug dispensation to the healthcare system. For instance, with regards to accessibility of drugs and services, drug dispensing systems such as the cyclical dispensing at the Christie NHS Foundation Trust that are integrated into the care process should not only ensure effective drug dispensation but also improve access to healthcare services in all levels of care by identifying and meeting their demands and needs.
However, it is important to note that access to dispensing services differs from access to drugs because as opposed to the latter, the former involves modifying institutional relations within the service network through social technologies (Stevens, Sinkin and Notter). This implies that to guarantee access to medication, the dispensing unit must consider various elements of accessibility such as the location of delivery, the patients’ conditions when entering the dispensing facilities as well as the impact of bureaucratic rules (Ghimire, Nepal and Bhandari). Such considerations are important because they limit opportunities and influence both collective and individual treatment decisions while stimulating user criticism. In case a patient’s access to drug dispensation is inhibited by accessibility to the dispensing facility, the pharmacy should make efforts to overcome the barriers even if it entails changing the organizational technical routines or modifying existing policies of practice (Filippini, Heimsch and Masiero). In the current study, it would be interesting to find out how the Christie NHS Foundation Trust makes such considerations in their implementation of cyclical dispensing.
Cost Minimization
The financial costs of cancer management, also termed as “financial toxicity of cancer” has recently been on an increasing trend due to a variety of factors including increased out of pocket expenditure on prescription drugs, lost financial income for being under cancer treatment, as well as the indirect costs incurred by caregivers (Stahl). Furthermore, as insurance companies struggle to cover the expenses of drugs and treatment, they shift those costs to the patients (Siddiqui and Rajkumar). This implies that any drug dispensing system adopted by cancer care facilities must consider the cost burden on patients and how patents can be enabled to reduce the overall cost of cancer treatment.
In the past few decades, the popularity of oral cancer drugs has increased, with most patients receiving a combination of chemotherapies consisting of both oral and intravenous drugs with the similar regimen, or as single therapies delivered within a sequence of different lines of therapy. The different modalities of treatment raise cost issues, and therefore treatment facilities must make more cost considerations in cancer drug dispensing than before (Faden, Chalkidou and Appleby).
Consequently, treatment facilities shave come up with innovative ways of drug dispensing that minimize costs while delivering similar quality of treatment as conventional methods. For instance, the use of in-office dispensing (IOD) compared to mail order dispensing in terms of cost-effectiveness (Kantarjian, Fojo and Mathisen). The latter dispensing technique will be of interest to the current study because the cyclical dispensing at the Christie NHS Foundation Trust also uses mail order pharmacy model. Community oncology services have adopted a trend of establishing IODs or retail pharmacies that are offered within the oncologist’s practice or from alternative retail pharmacies – both approaches allowing a direct dispensation of drugs to cancer patients by oncologists while enhancing the oncologist’s close monitoring and management of the patient (Danzon and Taylor). Before the adoption of the IOD trend, most oral cancer drugs were offered through mail order or specialty pharmacies. However, as opposed to IOD, mail order services enable the provision of additional services such as outcome measures, cost savings, adherence oversight, waste management and general disease management (Carles, Vilaprinyo and Cots). In the present study, it would be interesting to find out whether the cyclical dispensing at the Christie NHS Foundation Trust facilitates the delivery of these additional services thereby reducing costs while increasing value for patients’ money.
Methodology
A well-developed research methodology facilitates an effective interrogation and evaluation of research findings with respect to the procedures that led to the achievement of the research objectives and the reliability of those procedures. Therefore, it is important to describe the techniques and instruments used in a study.
The main aim of this chapter is to describe the research design, approaches, tools and techniques used to execute this study. It identifies important elements of research procedure including sampling, data collection, data analysis and ethical consideration made during the entire project. More importantly, this chapter gives a comprehensive justification of the various methods and techniques used herein, backed by existing literature on research methods.
Research Approach
Based on the assertions by Clark (2011), research approach refers to the systematic strategies and techniques used to gather data, assess the data and transform them into information that can be used to answer the research questions or conclusively achieve the research objectives. The descriptive framework adopted by the researcher to achieve the aims and objectives of the study. It involves a process that consist of data collection, analysis and formulation of conclusions based on the evidence gathered from the data (Jacobsen, 2017).
Two dimensions are usually taken when describing the research approach namely data collection approaches and data analysis approaches (Sutton & Austin 2015). With respect to data collection, according to Cronin et al (2014), a study can either be quantitative, qualitative or mixed approaches. While quantitative approaches have gained much popularity in the recent past, mixed approaches have also been proven to be effective in achieving similar objectives (Jacobsen, 2017). In this regard, Cronin et al (2014) asserts that qualitative approaches involve an in-depth understanding of the problem form the perspective of people experiencing. On the other hand, quantitative approaches entail the understanding of interlinkages between variables by using empirical data to identify trends and generate hypotheses (Lapn et al, 2012). A significant advantage of quantitative approaches is that quantitatively collected data can easily be measured and validated (Sutton & Austin 2015) while it is disadvantageous in the sense that they sum up various aspects of the underlying variables into a single measurement, making it difficult to effectively evaluate their components (Katon et al, 2011). On the other hand, one advantage of qualitative approaches is that they enable researchers to understand aspects of the data that cannot be understood in figure, thereby facilitating a better understanding of observed results (Jacobsen, 2017).
Against this background, the current study used quantitative research approach, particularly to facilitate the understanding of costs associated with the cyclical dispensing at the Christie NHS Foundation Trust. Participants received Likert-scaled questionnaires that would statistically be analysed.
With regards to data analysis approaches, a study can either use an inductive or deductive research approach. Inductive approach is described as the development of new hypotheses or theory-based research findings while deductive approaches seek to establish the validity of existing theories or frameworks. However, both approaches involve the collection of either primary or secondary data that are further analysed to develop comprehensive conclusions over the research topic (Jacobsen, 2017). As earlier mentioned, the main aim of this study is to identify the safety and cost effectiveness of cyclical dispensing at the Christie NHS Foundation Trust. It aims to achieve this by collecting both secondary and primary data and analysing that data to establish whether the cyclical dispensing at the Christie NHS Foundation Trust is safe and cost effective. Thus, the study used deductive approaches.
Research Design
Cronin et al (2014) describes research design as the general approach taken by a researcher to logically combine different aspects of a study to facilitate an effective answering of the research question. It integrates the various elements (e.g. data collection, analysis and measurement) of the study into a single framework that makes it easier for the researcher to develop and maintain the direction which the study would take (Mitchell, 2013).
Based on the research problem: to investigate the safety and effectiveness of cyclical dispensing at the Christie NHS Foundation Trust, the study adopted a descriptive research design. According to Cronin et al (2014), descriptive research design facilitates the answering of questions such as when, what, where, how and who is associated with the problem under investigation, although it may not conclusively give the answer to why. It is mostly used when the researcher aims to gather information the current i.e. what exists with respect to the problem under investigation (Jacobsen, 2017). Therefore, because the aim of the study was to investigate the safety and effectiveness of cyclical dispensing at the Christie NHS Foundation Trust, descriptive research design was deemed fit because it would help describe how safe and cheap the NHS Trust’s cyclical dispensing is now.
There are several other theoretical underpinnings as to why descriptive research design was selected for this study. First, according to Cronin et al (2014), descriptive research design is useful in situations where limitations are well understood because it helps to enhance the focus of the study. There are several limitations of cyclical dispensing that have been identified by literature. Therefore, buy using descriptive research design, the author was able to keep an eye on these limitations and understand how they impact on costs. Descriptive research design was also useful in collecting large amounts of data Lapn et al (2012), considering that there are many patients receiving drugs under cyclical dispensing at the Christie NHS Foundation Trust.
Sampling
The main aim of this study was to evaluate the safety and cost effectiveness of cyclical dispensing at the Christie NHS Foundation Trust. Therefore, the author needed a sample population to include in the study as opposed to including the entire population under cyclical dispensing at the Christie NHS Foundation Trust. The author therefore selected non-probability sampling method to recruit participants.
Particularly, non-probability sampling was selected for the study because it would facilitate the collection of rich data (Mitchell, 2013). Under non-probability sampling, the study used purposive sampling, whereby the author relied on their own judgment when selecting the participants. The first reason why purposive sampling was considered appropriate is that the researcher wanted to access a specific subset of population (i.e. those receiving cancer drugs from cyclical dispensing at the Christie NHS Foundation Trust) and the selected participants fitted that profile.
Data Collection
In academic research, data collection is the process of gathering the necessary information for answering the research questions (Mitchell, 2013). According to Cronin et al (2014), it is an important component of the study because the reliability of study results depends on how reliable the data collection process was. In the current study, data collection involved both primary and secondary sources of data. Primary sources entailed online questionnaires administered through Survey Monkey (an online research platform) while qualitative data were derived from resources and documentation retrieved from Christie NHS foundation Trust archives.
Questionnaires
The questionnaires will be designed to achieve all the research objectives. For instance, to quantify where medication supply has been either avoided or requested in order to ensure the safe and correct use of medication by the patient, explore the frequency of medication supply and determine whether costs and medication wastage have been reduced, and to explore the frequency of reporting adverse drug reactions (ADRs) and side effects by patients.
The questionnaires were self-administered and ended up providing quantitative data for quantitative data analysis. The questionnaires were administered within a period of one month and each participant was asked to return their questionnaires as soon as they completed. It as estimated that each questionnaire would take 15-20 minutes to complete.
Online questionnaires are often associated with low response rates because the researcher is not physically available to encourage participation (Mitchell, 2013). Therefore, to ensure a good response rate, each participant was offered a token of appreciation in the form of an airtime scratch card that was embedded o each survey page and could only be accessed after answering all the question.
But there was thin line between issuing the token as amotivation and the ethical issue of coercion. This ethical issue has been a subject of discussion among researchers for a while now. For instance, Lapn et al (2012) argue that coercion involves forcefully or putting excess pressure on the patient to participate in the study. However, Cronin et al (2014) claims that incentivising participants is unethical because they should me motivated to participate through their own conviction upon reviewing the significance of the study and how their participation will contribute to the good of the society. Contrariwise, Cronin et al (2014) claims that incentivising participants is not bad so long as the incentive is presented as an appreciation and is not of a high value to influence recipients’ decision to participate. Based on these arguments, the researcher only offered 1-pound worth of airtime to participants and this was considered not too much to offer as a motivation.
Data analysis
Upon collecting data, the study used Windows 2010 excel data analytic tools to analyse and present the data Excel data analysis tool has widely been used by researchers across the world (Mitchell, 2013). It is a powerful data analysis tool that can be used to analyse and present both qualitative and quantitative data even by researchers with little skills in quantitative data analysis (Katon et al, 2011). Particularly, excel was chosen for this study due to its simplicity and the ease with which it facilitates quantitative data analysis. Moreover, its ready availability (for windows 10 users) made it convenient and cheap to apply.
Ethical Issues
In any research involving humans, researchers are obligated to make ethical considerations and sort pout any ethical issues that might arose during the study (Katon et al, 2011). Ethical considerations are not only advocated for by the Nursing and Midwifery Council (2015) but also by university ethics committees because of the need to ensure safety of the participants involved in the study.
Fundamentally, ethical considerations involve moral values and how to separate right from wrong within the study context (Mitchell, 2013). According to Cronin et al (2014), it defies the decision-making process that the researcher must go through to identify the safest and most appropriate research instruments, tools and techniques that will not harm the participants.
Therefore, in the current study, the researcher made several ethical considerations especially because the study needed an interaction and a relationship with the participants to achieve its objectives. They entailed various precaution measures meant to ensure safety and protect the participants’ interest at different points of the study.
First, before beginning the study, the researcher conducted an evaluation of the significance and relevance of the study to identify if it was really needed. Particularly, this evaluation was conducted because it would be a waste of time and resources to engage in an unjustified study (Katon et al, 2011).
Advocate of research ethics such as Lapn et al (2012) argue that gathering data without informing the participants is a serious ethical fault that is kin to fraud. Therefore, before asking participants to fill the questionnaires, the researcher ensured that each of them signed an informed consent form (Appendix X), which is a document that seeks participants’ own volition before participating. According to Cronin et al (2014), the consent form also ensures that participants understand various details of the study such as the research objective, why they should participate, justification of the study and the social benefit of the study. It also informs them of their freedom to withdraw from the study at their will.
The author also ensures that no participant was exposed to any harm through harassment, anxiety or embarrassment. To do so, the author conducted a thorough evaluation of any possible unethical practice that might be encountered during the study such as inappropriate use of data and gathering of unauthorized information from the participants.
In a broader context, the author obeyed the principles of beneficence, non-maleficence, justice, confidentiality and truthfulness. Regarding beneficence, the author ensured that the participants’ interests were protected by only doing the good with the study (Katon et al, 2011). Conversely, non-maleficence was observed by ensuring that any decision made during the study would not have any negative implication on the participant.
Even if participants were safeguarded from any harm, it is possible that they might still suffer certain psychological consequences (Mitchell, 2013). This implies that the researcher had to be sensitive in all aspects of the study including storage and dissemination of the study results. Therefore, based on the principles of non-maleficence and beneficence, all the data collected from the participants were stored in a password-protected data stick to prevent any non-authorised individual from accessing it. Besides, part of the information provided in the informed consent was how the study would be disseminated. Meanwhile, all the questionnaires were discarded after data analysis.
Other ethical considerations made by the researcher included seeking ethical approval from the university’s ethics committee, avoiding the use of names and personal data (e.g. email address, residential address or workplace) in the research report. Furthermore, the researcher maintained high standards of professional practice.
Results
One of the questions in the questionnaire that evaluate the safety of the cyclical dispensing at the Christie NHS Foundation Trust was whether patients made or avoided medication orders to ensure continuity with and safety of medication. Therefore, the researcher sought to know the expected date of medication supply for each respondent, whether they placed an order of that supply and whether that order correspondent with the correct date of supply. There was a 50% response (i.e. 25) to this question and 100% of the respondents agreed to have placed an order and received resupply in correspondence with the correct date of resupply. It was difficult to tell whether the other 50% of the respondents who did not answer this question observed the correct medication re-ordering.

Furthermore, all the respondents who reported to have observed a correct reordering of medication had no excess supply of medications at home. It would have been considered a safety hazard if they ordered for re-supply while still having some stored medication. Thus, it is highly likely that the respondents who observed correct reordering had completed their medication cycle on time and were ready to begin their next cycle.
Similarly, none of the respondents had been advised to stop medication, change a dosage or had their treatment interrupted at the time of ordering for a resupply. This means that they were correctly meant to proceed to the next cycle of medication. A possible implication of this finding is that most of the respondents requested for medication according to the cycle prescription and ordered for the next supply at the correct time when they were supposed to do so.
Frequency of medication supply
The cyclical dispensing at the Christie NHS Foundation Trust occurs after every month, whereby patients pick or have drugs delivered for them after approximately 30 days. Thus, the researcher was interested in evaluating the frequency with which the patients receives medication and how this frequency affects cost and safety. In this regard, the study found that a majority (92%) of the respondents received drugs significantly within one month after the reception of previous cycle’s dosage, with an allowance of at most two days before and after the 30th date. This implies that most of the respondents observed the frequency with which they were to receive new drugs, saved on costs by not receiving excess drugs and maintained their safety by taking prescribed amounts.
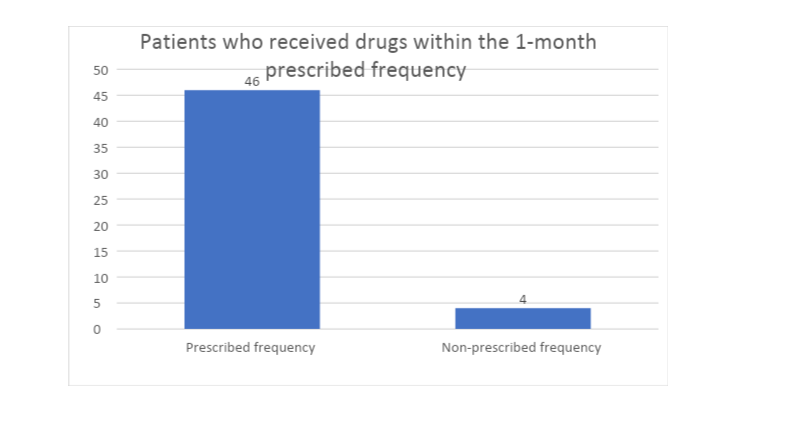
Thus, it is possible to extrapolate that the cyclical dispensing at the Christie NHS Foundation Trust maintains its frequency of supply and does not expose patients to cost and safety implications by interrupting the prescribed supply frequency.

Adverse drug reaction is a key safety element that both patients and practitioners must be on the look out for. In the current study, the author was interested in understanding whether there are any adverse drug reactions associated with cyclical dispensing at the Christie NHS Foundation Trust and the frequency with which such cases are reported. Besides, the study was interested in identifying any intervention measures put in place for patients that experience adverse drug reactions.
Results of the study indicated that only 6 of the respondents experienced drug reactions, even though not to an adverse level. All the 6 respondents sought practitioners’ immediate attention and received a change in prescriptions. No patient was at a severe health risk due to the reaction.


The low frequency and severity of drug reactions among the respondents point to the level of safety procedures maintained by Christie NHS Foundation Trust during prescriptions. While there are no explanations to the few cases of drug reactions, the significantly high number of patients who did not experience any drug reactions illustrate the due diligence observed in medical prescription by the Trust.
References
- Arlow, R., 2011. Chaplin v Royal Devon and Exeter Hospital NHS Foundation Trust. Ecclesiastical Law Journal, , 13(02), pp. 242-242.
- Belfrage, B., Koldestam, A., Sjöberg, C. & Wallerstedt, S. M., 2014. Prevalence of suboptimal drug treatment in patients with and without multidose drug dispensing—a cross-sectional study. European Journal of Clinical Pharmacology, , 70(7), pp. 867-872.
- Crowe, S. et al., 2010. Planned implementations of ePrescribing systems in NHS hospitals in England : a questionnaire study.. Jrsm Short Reports, , 1(4), pp. 33-33.
- Erning, F. v. et al., 2016. Drug dispensings among elderly in the year before colon cancer diagnosis versus matched cancer-free controls.. Journal of Clinical Pharmacy and Therapeutics, , 41(5), pp. 538-545.
- Fife, N., 2011. NHS Fife Board. [Online] Available at: https://nhsfife.org/nhs/index.cfm?fuseaction=nhs.pagegroup&p2sid=5ccf282b-9f18-af85-f4647c8fdb390dba&themeid=e44c37c3-5056-8c6f-c003cd63c15d8ff0 [Accessed 27 4 2020].
- Johnell, K. & Fastbom, J., 2008. Multi-dose drug dispensing and inappropriate drug use: A nationwide register-based study of over 700 000 elderly. Scandinavian Journal of Primary Health Care, , 26(2), pp. 86-91.
- Kulesskiy, E., Saarela, J., Turunen, L. & Wennerberg, K., 2016. Precision Cancer Medicine in the Acoustic Dispensing Era Ex Vivo Primary Cell Drug Sensitivity Testing. Journal of Laboratory Automation, , 21(1), pp. 27-36.
- Suda, K. J. et al., 2017. Opioid dispensing and overlap in veterans with non-cancer pain eligible for Medicare Part D. Journal of The American Pharmacists Association, , 57(3), pp. 333-340.
- Verger, P. et al., 2017. Psychotropic drug dispensing in people with and without cancer in France. Journal of Cancer Survivorship, , 11(1), pp. 92-101.
Continue your journey with our comprehensive guide to Strategies for Success Exploring Employee Retention.
- 24/7 Customer Support
- 100% Customer Satisfaction
- No Privacy Violation
- Quick Services
- Subject Experts



